Zika virus: Difference between revisions
| Line 76: | Line 76: | ||
<references> | <references> | ||
"CDC Issues Guidelines for Pregnant Women during Zika Outbreak." Reuters. Thomson Reuters, 19 Jan. 2016. Web. 15 Apr. 2016. | |||
Created by Derek Lehman, student of Tyrrell Conway at the University of Oklahoma. | Created by Derek Lehman, student of Tyrrell Conway at the University of Oklahoma. | ||
Revision as of 19:32, 15 April 2016

Outbreak 2015
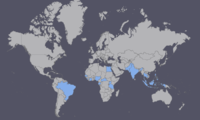
In May 2015 an outbreak of Zika virus began in Brazil and spread to much of Central and South America. The spread of the virus led to reports of health problems such as Guillain-Barré syndrome and possibly microcephaly, a disease in which babies are born with abnormally small heads and brains that have not developed properly. The WHO said a direct causal relationship between Zika virus infection and birth defects has not yet been established but is strongly suspected. However, Dr. Tom Frieden, director of the U.S. Centers for Disease Control and Prevention (CDC) reports that "There isn't any doubt that Zika causes microcephaly." As a result, the CDC issued travel advisories for those traveling to affected areas.
Baltimore Classification and Taxonomy
Group IV: (+) sense single-stranded RNA virus
| Order = Unassigned
| Family = Flaviviridae
| Genus = Flavivirus
| species = Zika virus
{|NCBI: Taxonomy Genome: Genome|}
Description and Significance

Zika virus (ZIKV) is a mosquito-borne flavivirus that was first isolated from a rhesus monkey in the Zika forest of Uganda in 1947. [2] In 1968, isolation from human hosts occurred in residents of Nigeria. [3] Since then, multiple studies have confirmed ZIKV antibody in humans from a multitude of countries in Africa and parts of Asia. [3] In 2015, ZIKV first appeared outside of Africa and Asia when it was isolated in Brazil where is has caused a minor outbreak following the 2014 FIFA World Cup. [4] ZIKV is closely related to other mosquito-borne flaviviruses such as the dengue, yellow fever, West Nile, and Japanese encephalitis viruses. [3] ZIKV causes a disease known as Zika fever, which is characterized by a macropapular rash covering the body, fever, joint pain, and malaise. [3] Although there have yet to be serious complications arising from ZIKV, it's appearance across the globe, mosquito-driven transmission cycle, and possible spread via sexual contact make ZIKV an important emerging pathogen whose global impact is yet to be discovered.
Genome Structure
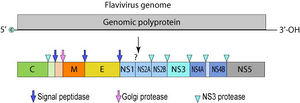
The Zika virus is a positive sense single-stranded RNA molecule 10794 bases long [2] with two non-coding regions flanking regions known as the 5' NCR and the 3' NCR. The open reading frame of the Zika virus reads as follows: 5′-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5-3′ and codes for a polyprotein that is subsequently cleaved into capsid (C), precursor membrane (prM), envelope (E), and non-structural proteins (NS). [5] The E protein composes the majority of the virion surface and is involved with aspects of replication such as host cell binding and membrane fusion. [2] NS1, NS3, and NS5 are large, highly-conserved proteins while the NS2A, NS2B, NS4A, and NS4B proteins are smaller, hydrophobic proteins. [5] Located in the 3' NCR are 428 nucleotides that may play a part in translation, RNA packaging, cyclization, genome stabilization, and recognition. [2] The 3' NCR forms a loop structure and the 5' NCR allows translation via a methylated nucleotide cap or a genome-linked protein. [6]
Virion Structure of a Zika virus
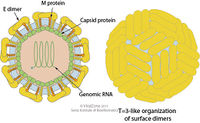
The structure of ZIKV follows that of other flaviviruses. It contains a nucleocapsid approximately 25-30 nm in diameter surrounded by a host-membrane derived lipid bilayer that contains envelope proteins E and M. The virion is approximately 40 nm in diameter with surface projections that measure roughly 5-10 nm. [5] The surface proteins are arranged in an icosohedral-like symmetry. [6]
Reproductive Cycle of a Zika virus in a Host Cell
The reproductive cycle of ZIKV follows that of other known flaviviruses. First, the virion attaches to the host cell membrane receptors via the envelope protein which induces virion endocytosis. Next, the virus membrane fuses with the endosomal membrane and the ssRNA genome of the virus is released into the cytoplasm of the host cell. It is then translated into a polyprotein that is subsequently cleaved to form all structural and non-structural proteins. Replication then takes place at intracellular compartments known as cytoplasmic viral factories in the endoplasmic reticulum resulting in a dsRNA genome. The dsRNA genome is then transcribed resulting in additional ssRNA genomes. Assembly then occurs within the endoplasmic reiticulum and the new virions are transported to the Golgi apparatus and then excreted into the intracellular space where the new virions can infect new host cells. [6]
Viral Ecology & Pathology
Epidemiology
Since it's first detection in Uganda, ZIKV has appeared in the following countries of Africa and Asia: Gabon, Egypt, Nigeria, Senegal, Sierra Leone, Côte d'Ivoire, Central African Republic, Cambodia, Micronesia, Malaysia, Pakistan, India, Thailand, Philippines, and Indonesia. [2] ZIKV has also presented itself in the Western world in 2015 when it was isolated in Brazil, likely a result of the 2014 World Cup. [4]
Transmission
The transmission of ZIKV is predominately through the use of mosquitoes as vectors. However, a recent case shows that human-to-human contact is possible, which would make it the first time an insect-borne disease was passed via sexual contact. [7] In nature, reservoirs for ZIKV virus are restricted to primates. [3] However, one study showed antibody to ZIKV in rodents. [8] Due to the oddity of transmission through sexual contact, further studies should be conducted to identify all possible reservoirs and insect vectors to further understand ZIKV transmission.
Mosquito-borne
Transmission of ZIKV in nature is thought to follow that of other closely related flaviviruses. This transmission includes a mosquito vector and a mammalian host. [9] For ZIKV, a variety of mosquitoes from the genus Aedes are viable vectors.These include A. africanus, A. aegypti, A. vitattus, A. furcifer, A. apicoargenteus, and A. luteocephalus. [3] Horizontal transmission is the terminology given to transmission of viruses from mosquito vectors to mammalian hosts. On the contrary, vertical transmission is the result of mosquito populations passing on the virus to their offspring. Transmission occurs when an infected vector feeds on a host. [9] Studies show that the incubation time for ZIKV in mosquito vectors is approximately 10 days. [3]
Human to Human
After returning from a research mission to Senegal, Dr. Brian Foy of the University of Colorado appeared to have passed ZIKV to his wife after sexual intercourse in 2008. Both Dr. Foy and his Ph.D. student Kevin Kobylinski were bitten by numerous mosquitoes while abroad and became ill about 5 days after returning to the U.S. Both showed signs of Zika fever and Dr. Foy's wife saw what she believed to be blood in his semen. Approximately 10 days later, Foy's wife showed signs of Zika fever while their four children remained healthy. At first it was believed that Foy had contracted dengue fever, but after Kobylinski had an encounter with medical entomologist Andrew Haddow who had experience with ZIKV, tests were run that confirmed all three patients possessed antibody for ZIKV. Circumstantial evidence suggests that sexual contact was the mode of transmission due to the high unlikeliness that Foy's wife was bitten by the tropical Aedes mosquito in Colorado. Additionally, the virus requires a 2-week life cycle in mosquito hosts before infecting humans and Foy's wife showed symptoms merely 10 days upon his return. [7]
In addition to sexual contact, studies have shown that ZIKV can reside in blood transfusions and they can be a possible vector for human-to-human transmission. [10]
Zika Fever and ZIKV Pathogenesis
Zika fever is the disease associated with ZIKV infection. While there are scarce resources describing ZIKV pathogenesis exclusively, the general consensus is that mosquito-borne flaviviruses replicate initially in the dendritic cells at the site of infection where it then spreads to other areas of the body via the bloodstream and lymphatic system. Viral replication typically occurs in the cytoplasm of infected cells, but there is a study that shows ZIKV antigens can also be found in the nuclei of infected host cells. [3]
Symptoms

The first well-documented case of Zika fever in 1964 describes symptoms as beginning with a headache and then continuing to include a maculopapular rash covering a significant portion of the body the following day. In addition, a fever was present as well as back pain and a general feeling of malaise. In 1973, a patient with Zika fever presented with fever, joint pain, and headache but the rash was absent. Another outbreak in Indonesia resulted in all 7 victims showing fever, but varying additional symptoms ranging from abdominal pain and dizziness to diarrhea and anorexia but no signs of rash. The outbreak on Yap Island showed the maculopapular rash, joint pain, and conjunctivitis. In all cases, symptoms were mild and typically self-resolved in the course of a week without the need for hospitalization or risk of severe complications. [3]
Diagnosis
Diagnosis for ZIKV infection include PCR tests to detect viral RNA as well as additional tests to detect ZIKV antibody (IgM) in serum. IgM for ZIKV is typically detectable around 3-5 days after infection, but cross-reactivity with closely related dengue, yellow fever, Japanese encephalitis, and West Nile viruses are possible. These cross-reactive results were more common in patients that denoted signs of previous flavivirus infection than patients with primary ZIKV infection. PCR tests should be conducted within 10 days of onset of illness. For best diagnosis practices, serum samples should be analyzed as early as possible with a second test 2 to 3 weeks after that. [3]
Treatment and Prevention
Presently, there is no vaccine for ZIKV or treatment for Zika fever. Because symptoms are relatively mild and the disease is self-limiting, only supportive therapy is employed. Patients with Zika fever should remain hydrated and rest. Acetaminophen can be prescribed to combat fever. [11]
Given that ZIKV is transmitted by mosquitoes, it is advocated by the CDC to use insect repellent, long sleeved-clothing, and intervention in reducing the number of mosquito vectors. [3] Additionally, due to recent studies that show the possibility of sexual transmission, safe sex practices are also encouraged.
Damage Response Framework
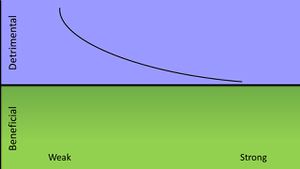
As the understanding of microbial pathogenesis increases, so must our ways of classifying pathogeneis patterns. An emerging method of classification known as the damage response framework seeks to incorporate the full scope of the host-pathogen interaction by gauging the effect on the host as a function of damage/benefit versus host immune response. This method of classification takes into account that microbial infection doesn't always result in disease for host with healthy immune systems, but can for immunocompromised individuals. Additionally, the damage response framework method of classification explores the possibility that colonization by a certain microbial species could provide a benefit to the host so long as the microbial population is kept in check. [12] A horizontal line down the middle of the graph represents an asymptomatic infection where the host is neither damaged or receives any benefit. If the trend moves toward the top of the graph the host is experiencing damage while if the trend migrates toward the bottom of the graph the host and pathogen are in a state of commensalism.
For Zika virus, there is no known benefit to the host as a result of infection but not all infections result in Zika fever. Therefore, the damage response framework would look something similar to a curve in which damage occurs at a low host immune response and no damage occurs at a high host immune response. This is considered to be a Class 2 pathogen.
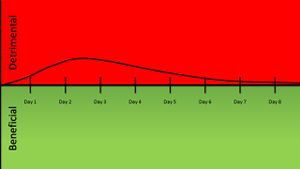
Another way to look at ZIKV infection would be to plot a chart of damage versus time. In doing this, the course of symptomatic ZIKV infection can be analyzed. The infection peaks around day two or three when symptoms peak, and then gradually taper off as the infection naturally clears in the course of approximately a week.
References
<references>
"CDC Issues Guidelines for Pregnant Women during Zika Outbreak." Reuters. Thomson Reuters, 19 Jan. 2016. Web. 15 Apr. 2016.
Created by Derek Lehman, student of Tyrrell Conway at the University of Oklahoma.
- ↑ http://www.cdc.gov/zika/
- ↑ 2.0 2.1 2.2 2.3 2.4 Faye O, Freire C, Iamarino A, Faye O, de Oliveira J, Diallo M, Zanotto P, Sall A. (2014) Molecular Evolution of Zika Virus during Its Emergence in the 20th Century. PLOS Neglected Tropical Diseases
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 Hayes E. (2009) Zika Virus Outside Africa. CDC Emerging Infectious Diseases
- ↑ 4.0 4.1 Campos GS, Bandeira AC, Sardi SI. (2015) Zika virus outbreak, Bahia, Brazil. CDC Emerging Infectious Diseases
- ↑ 5.0 5.1 5.2 Chambers T, Hahn C, Galler R, Rice C. (1990) Flavivirus genome organization, expression, and replication. Annual Reviews of Microbiology
- ↑ 6.0 6.1 6.2 Flaviviridae. Swiss Institute of Bioinformatics
- ↑ 7.0 7.1 Enserink M. (2011) Sex After A Field Trip Yields Scientific First. Science Magazine
- ↑ Darwish M, Hoogstraal H, Roberts T, Ahmed I, Omar F. (1983) A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. PubMed
- ↑ 9.0 9.1 Yan-Jang H, Higgs S, Horne K, Vanlandingham D. (2014) Flavivirus-Mosquito Interactions. PubMed
- ↑ Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, Shan Yan A, Cao-Lormeau VM, Broult J. (2014) Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. PubMed
- ↑ Oakley A. (2014) Zika virus. DermNet NZ
- ↑ Casadevall A, Pirofski L. (2003) The damage-response framework of microbial pathogenesis. PubMed