Borrelia burgdorferi and Lyme Disease Pathogenesis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
==Section 1: Overview of Borellia Burgdorferi == | ==Section 1: Overview of Borellia Burgdorferi == | ||
[[Image:Borrelia_Pic.jpeg|thumb|300px| | [[Image:Borrelia_Pic.jpeg|thumb|300px|left|Figure 1. A scanning electron micrograph of a cluster Borrelia burgdorferi bacteria. Micrograph taken by Claudia Molins at the CDC in 2011. [http://phil.cdc.gov/phil/details.asp?pid=13176}.]] | ||
[[Image:Spirochete_Morphology.jpeg|thumb|300px|left| Figure 2. Diagram of spirochete showing the flagella and the flagellar motor in between the outer and inner membrane. [http://www.sciencedirect.com/science/article/pii/S1369527415001368]] | |||
| Line 37: | Line 35: | ||
Borellia Burgendorferi is a spirochete bacterium. Like many spirochete species it is very small, with a width of about .3 μm and a length of about 5-20 μm. B. Burgendorferi shares the typical spirochete morphology with an outer membrane surrounding an inner membrane. In between these two membranes there are flageller filaments that wrap around the cell and are rotated via a flageller motor to propel the bacterium in a corkscrew motion (Figure 2). It is believed that this corkscrew motion helps the bacterium move through highly viscous environments [1]. The bacterium has a very slow replication rate, which is the one of the reasons it is very hard to identify infection based on blood cell levels [2]. It is also believed that this slow replication time might assist with the bacterium avoiding the host immune response but that will be covered below. | |||
==Section 2: Lyme Disease Overview== | |||
[[Image:Lyme Cycle.jpeg|thumb|300px|right| Figure 3. Diagram of B. burgendorferi life cycle and transmission from tick host to other organisms. [http://www.nature.com/nrmicro/journal/v10/n2/fig_tab/nrmicro2714_F1.html]] | [[Image:Lyme Cycle.jpeg|thumb|300px|right| Figure 3. Diagram of B. burgendorferi life cycle and transmission from tick host to other organisms. [http://www.nature.com/nrmicro/journal/v10/n2/fig_tab/nrmicro2714_F1.html]] | ||
| Line 67: | Line 46: | ||
Lyme disease was first noticed by Doctor Alan Steere in 1977, when he noticed that persons were being infected by an unknown pathogen producing the same symptoms in the same geographical region and during the same times of the year. It was finally discovered that the infection was due to spirochete bacteria found in the midgut of ticks local to the area. The infection was found to create a rash and the bacteria was named Borellia Burgendorferi. Ticks were found to have taken in the bacteria while still larvae and feeding on rodent carriers. Once these ticks grow up into their nymph and adult stages they feed on all species of larger hosts, thus transmitting the bacteria on. They also will feed on more rodents, some who aren’t infected, hence perpetuating the issue (Figure 3). Once a tick bites a human host B. Burgendorferi bacteria migrate to the salivary glands of the ticks and into the blood stream of the new host. The bacteria will then travel through the blood stream of the host, eventually settling in various tissues and causing an inflammatory response and damage [3]. | |||
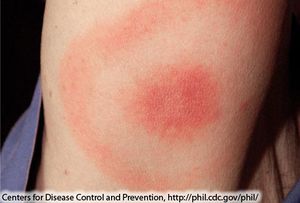
B. Burgendorferi does not actually contain any virulence factors and the damage done is due to necessary steps for the bacterium to replicate. The damage done is actually due to a stimulation of the immune system, which attacks, damaging the tissue around the bacteria. This response by the immune system is actually what causes the symptoms of Lyme disease, and is the reason why the symptoms can vary so much due to the fact that the areas damaged by the immune system will create varying symptoms [3]. For example if the ganglia and glial cells are damaged then the host will suffer from neurological symptoms like tremors, arthritis, or seizures, and pain [4]. A local immune response at the site of infection can cause a erythema migrans (EM) rash (Figure 4) [5]. | |||
| Line 122: | Line 66: | ||
One of the host’s immune responses to an infection is to limit the production and concentration of iron in the body. This is due to many cells, including pathogenic bacteria, requiring iron for the production of enzymes essential to growth. By limiting the iron levels, and the ability for bacteria to grow, the body can possibly get the upper hand on fighting the infection. This is done by the release of the hormone hepciden by the liver, which lowers iron levels. This makes the person anemic which is why people feel tired and weak when they are sick [6]. Researchers at the Indiana University School of Medicine found that B. Borgendorferi has eliminated this issue by substituting manganese for iron in making the enzyme. | |||
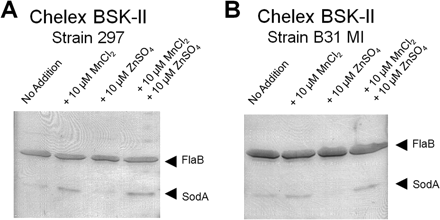
This idea was first suggested when it was found that B. Borgendorferi was unable to transport iron across its membrane and had very low levels of intercellular iron. It was reasonable to assume then that the bacteria did not rely on iron but some other metal that they had adapted to using. They found that changing the extracellular manganese concentrations affected the expression levels of the protein SodA, responsible for protecting the anaerobic bacteria from oxygen [7]. The expression level was also hindered by the addition of extracellular zinc to the mixture (Figure 5). | |||
The authors found some more interesting discoveries about these pathways. They found that when growth in an environment of streptonigrin, an antibiotic, the bacterial growth was highly limited. The effects of streptonigrin were lessoned when manganese was added to the environment. It is believed that the binding of manganese to the SodA protein limits the effect of the antibiotic. | |||
With all of these results in mind it points to a possible treatment for Lyme disease that could be researched into. Although the use of manganese hides the bacteria from the host’s immune response of limiting iron availability, it also creates a specific place to attack the bacteria. If manganese can be removed or limited to the bacteria it could slow or even stop growth. Further it was found that the addition of zinc would possibly increase the effects of antibiotics on the bacterial infection. Further research would have to be done but it could be a promising start for Lyme disease treatment. | |||
Revision as of 00:29, 27 April 2016
Research Question: Why is the bacterium so good at evading the host's immune response? [1]
Section 1: Overview of Borellia Burgdorferi
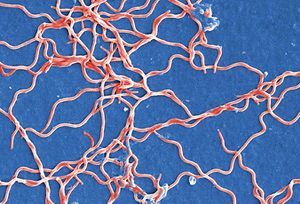
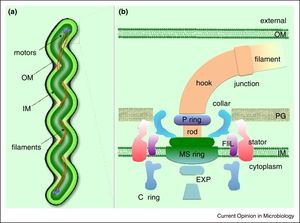
Borellia Burgendorferi is a spirochete bacterium. Like many spirochete species it is very small, with a width of about .3 μm and a length of about 5-20 μm. B. Burgendorferi shares the typical spirochete morphology with an outer membrane surrounding an inner membrane. In between these two membranes there are flageller filaments that wrap around the cell and are rotated via a flageller motor to propel the bacterium in a corkscrew motion (Figure 2). It is believed that this corkscrew motion helps the bacterium move through highly viscous environments [1]. The bacterium has a very slow replication rate, which is the one of the reasons it is very hard to identify infection based on blood cell levels [2]. It is also believed that this slow replication time might assist with the bacterium avoiding the host immune response but that will be covered below.
Section 2: Lyme Disease Overview
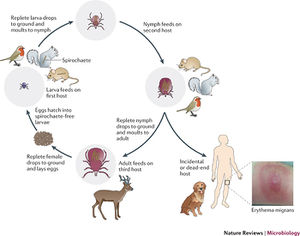
Lyme disease was first noticed by Doctor Alan Steere in 1977, when he noticed that persons were being infected by an unknown pathogen producing the same symptoms in the same geographical region and during the same times of the year. It was finally discovered that the infection was due to spirochete bacteria found in the midgut of ticks local to the area. The infection was found to create a rash and the bacteria was named Borellia Burgendorferi. Ticks were found to have taken in the bacteria while still larvae and feeding on rodent carriers. Once these ticks grow up into their nymph and adult stages they feed on all species of larger hosts, thus transmitting the bacteria on. They also will feed on more rodents, some who aren’t infected, hence perpetuating the issue (Figure 3). Once a tick bites a human host B. Burgendorferi bacteria migrate to the salivary glands of the ticks and into the blood stream of the new host. The bacteria will then travel through the blood stream of the host, eventually settling in various tissues and causing an inflammatory response and damage [3].
B. Burgendorferi does not actually contain any virulence factors and the damage done is due to necessary steps for the bacterium to replicate. The damage done is actually due to a stimulation of the immune system, which attacks, damaging the tissue around the bacteria. This response by the immune system is actually what causes the symptoms of Lyme disease, and is the reason why the symptoms can vary so much due to the fact that the areas damaged by the immune system will create varying symptoms [3]. For example if the ganglia and glial cells are damaged then the host will suffer from neurological symptoms like tremors, arthritis, or seizures, and pain [4]. A local immune response at the site of infection can cause a erythema migrans (EM) rash (Figure 4) [5].
Section 3: B. Borgendorferi use of magnesium to avoid immune response
One of the host’s immune responses to an infection is to limit the production and concentration of iron in the body. This is due to many cells, including pathogenic bacteria, requiring iron for the production of enzymes essential to growth. By limiting the iron levels, and the ability for bacteria to grow, the body can possibly get the upper hand on fighting the infection. This is done by the release of the hormone hepciden by the liver, which lowers iron levels. This makes the person anemic which is why people feel tired and weak when they are sick [6]. Researchers at the Indiana University School of Medicine found that B. Borgendorferi has eliminated this issue by substituting manganese for iron in making the enzyme.
This idea was first suggested when it was found that B. Borgendorferi was unable to transport iron across its membrane and had very low levels of intercellular iron. It was reasonable to assume then that the bacteria did not rely on iron but some other metal that they had adapted to using. They found that changing the extracellular manganese concentrations affected the expression levels of the protein SodA, responsible for protecting the anaerobic bacteria from oxygen [7]. The expression level was also hindered by the addition of extracellular zinc to the mixture (Figure 5).
The authors found some more interesting discoveries about these pathways. They found that when growth in an environment of streptonigrin, an antibiotic, the bacterial growth was highly limited. The effects of streptonigrin were lessoned when manganese was added to the environment. It is believed that the binding of manganese to the SodA protein limits the effect of the antibiotic.
With all of these results in mind it points to a possible treatment for Lyme disease that could be researched into. Although the use of manganese hides the bacteria from the host’s immune response of limiting iron availability, it also creates a specific place to attack the bacteria. If manganese can be removed or limited to the bacteria it could slow or even stop growth. Further it was found that the addition of zinc would possibly increase the effects of antibiotics on the bacterial infection. Further research would have to be done but it could be a promising start for Lyme disease treatment.
Section 4: Tick saliva immunosuppressant
Section 5: B. burgendorferi escaping the blood stream
Section 6: Borrelia burgendorferi resistance to antibiotics
Section 7: Borrelia burgendorferi Outer Surface Proteins and possible vaccine
Section 8: Current Research
References
- ↑ [1]=Troxell, Bryan, Haijun Xu, and Frank Yang. "Borrelia Burgdorferi, a Pathogen That Lacks Iron, Encodes a Manganese-dependent Superoxide Dismutase Essential for Resistance to Streptonigrin." Borrelia Burgdorferi, a Pathogen That Lacks Iron, Encodes a Manganese-dependent Superoxide Dismutase Essential for Resistance to Streptonigrin. Journal of Biological Chemistry, 12 Apr. 2012. Web. 17 Apr. 2016.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.
