User:S4414057: Difference between revisions
| Line 87: | Line 87: | ||
Therefore, in this research, it was observed that a range of fibroblast-derived inflammatory mediators were inactivated by ''P. gingivalis'' as a result of gingipain proteolytic activities <sup>[[#References|[12]]]</sup>. | Therefore, in this research, it was observed that a range of fibroblast-derived inflammatory mediators were inactivated by ''P. gingivalis'' as a result of gingipain proteolytic activities <sup>[[#References|[12]]]</sup>. | ||
To conclude, all of these new discoveries aid in the increased understanding of the innate and acquired immunity interaction between the periodontal bacteria and the host. | To conclude, all of these new discoveries aid in the increased understanding of the innate and acquired immunity interaction between the periodontal bacteria and the host. It allowed the creation of new strategies and cures for the prevention and treatment of periodontitis and other related chronic inflammatory diseases. | ||
==References== | ==References== | ||
Latest revision as of 10:59, 23 September 2016
Chen Yanwen Bench E 31 August 2016 MICR3004
Classification
Higher order taxa
Bacteria (Kingdom) – Bacteria (Domain) – Bacteroidetes (Phylum)– Bacteroidia (Class)– Bacteriodales (Order) – Porphyromonadaceae (Family) – Porphyromonas (Genus)
Species
Porphyromonas gingivalis. Type strain: Strain 2561 = ATCC 33277= CCUG 25893 = CCUG 25928 = CIP 103683 = DSM 20709 = JCM 12257 = NCTC 11834.
Description and significance
Prophyromonas gingivalis was first discovered in 1980 and formally known as Bacteriodes gingivalis before being re-classified as a new genus, Prophyromonas, in 1988.
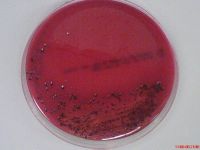
Prophyromonas gingivalis, P. gingivalis, is a non-motile, asaccharolytic, gram-negative obligatory anaerobic rod-shaped bacterium which forms black-pigmented colonies when grown in blood agar [1]. Blood agar was used for culturing P. gingivalis as it possesses an absolute requirement for iron for its growth.
Additionally, P. gingivalis is a type of major causative agent in the initial induction and progression of multiple types of periodontal diseases [2]. It was observed to be present in 85.75% of the subgingival plaque obtained from chronic periodontitis affected victims [1].
P. gingivalis is commonly found in the subgingival sulcas of the human oral cavity [1]. Due to the nature as an obligate anaerobe, P. gingivalis act as a secondary colonizer to adhere to primary colonizers such as Streptococcus gordonii. Furthermore, the obligatory anaerobe was associated with T. denticola and T. forsynthia to form a red bacterial complex which was uniquely recognized in severely advanced periodontal lesions [1]. Over the past few decades, invasive and non-invasive strains were classified based on its abscess forming ability in mouse models [1]. Invasive strain of P. gingivalis demonstrated higher pathogenic activities in both in vitro and in vivo, as compared to non-invasive strains.
Severe P. gingivalis infections were found leading to periodontal diseases. Periodontal disease involves the pathological inflammatory state of the gingiva and the surrounding structures of the periodontium. Periodontitis is a severe form of periodontal disease as formation of irreversible plaque-induced inflammation of the periodontal tissues causes periodontal ligament destruction. Periodontitis were observed to increase the risk for multiple severe conditions such as cardiovascular diseases and diabetes [1]. Hence, with the increased frequency of P. gingivalis found in periodontitis lesions, P. gingivalis was suspected to synthesize numerous pathogenic factors, resulting in increasing disease severity. It is important to study this pathogen due to its ability to implicate health issues and the lack of knowledge in destructive biological molecule destruction.
Genome structure
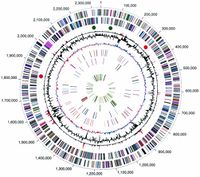
Presently, a complete genome sequence of the oral pathogenic bacterium P. gingivalis, strain W83, is available. The genome of W83 has a single circular chromosome of 2 343 476 bp, consisting of an average G+C content of 48.3% [3]. A grand total of 1,990 ORFs were identified in the genome. Four ribosomal operons were identified; 5S-23S-tRNAAla-tRNAIle-16S [3]. Additionally, 2 structural RNA gene and 53 tRNA genes specific for all 20 amino acids were observed.
Cell structure and metabolism
P. gingivalis is a asaccharolytic, non-motile, Gram-negative, rod-shaped, obligatory bacterium that forms black-pigmented colonies on blood agar [1]. Before the commencement of colonization, P. gingivalis has to overcome the external protective barriers of the host. Colonization of host tissues requires virulent factors such as fimbriae, capsules, lipopolysaccharide (LPS), lipoteichoic acids, hemagglutinins, gingipains, outer membrane proteins and outer membrane vesicles [1].
Microorganisms had to adhere to the teeth or mucosal surfaces first to initiate bacterial localization in the oral cavity. P. gingivalis utilizes a major outer membrane protein, fimbriae, to significantly adhere to host tissues. Furthermore, when P. gingivalis is growing in a subgingival biofilm under strict anaerobic conditions, the obligatory anaerobe is largely dependent on gingipain for the source of carbon and nitrogen. Gingipains were also utilized in evading host defences by degrading antibacterial peptides such as complement factors and T cell receptors.
P. gingivalis are generally non-motile when they are periodontal pathogens. However, during the initial stages of infection, it utilizes T9SS-related genes such as porK, porL, porM, porN, sov, porW and porT respectively for gliding motility [5]. Due to the nature of P. gingivalis being an asaccharolytic bacterium, it cannot only be dependent on glucose or carbohydrates alone [6]. P. gingivalis was observed to obtain its energy from the metabolism of amino acids.
Furthermore, P. gingivalis was found to have a higher preference to aspartate/asparagine amino acids. P. gingivalis metabolism produces multiple short chain fatty acid byproducts which are cytotoxic and were found to have a shift in production between the compounds, based on the different growth conditions provided [6]. Also, due to it absolute iron requirement for growth, P. gingivalis was observed to be capable of storing hemins on the cell surface. Hence, the characteristic black-colored pigments produced was actually due to hemin accumulation caused by the bacterium [6].
Ecology
P. gingivalis is an obligatory anaerobe. It is usually found in the orifice of humans. Within the oral cavity, it thrives in the subgingival sites and are usually found in the periodontal pockets [7].
The obligatory bacterium can also be found in the supragingival plaque, oral mucosal surfaces, dorsum of tongue, saliva and pharynx. Rarely, P. gingivalis may cause infections such as endocarditis and formation of abscesses in the lungs, head, neck and other abdominal areas [7]. Oddly, P. gingivalis do not seem to be able to colonize clean tooth surface. Instead, it favours areas with inflammation due to the lack of proper hygiene and sites thriving with gram-positive dental plaque bacteria.
P. gingivalis studies have observed that the periodontal pathogens most commonly cluster in families. Hence, suggesting that the bacteria may be transmitted between family members or that all the family members share the same susceptibility of P. gingivalis colonization [7]. There are several routes of transmission either via vertical, horizontal or person-to-person transmission. Vertical transmission is the transmission of organism from the parents to the children whereas horizontal transmission is the transmission which may occur between siblings or spouse. Lastly, infection could be transmitted from person to person via salivary or mucosal contact.
Pathology
P. gingivalis are capable of locally invading periodontal tissues and evading host defence mechanism [4]. This will lead to the eventual destruction of the surrounding tissues and tooth loss. To perform all these actions, it utilizes a wide array of virulence factors which results in the deregulation of the innate immune and inflammatory responses. As such, P. gingivalis often result in commonly known diseases such as gingivitis and periodontitis. Gingivitis is the inflammation of the gum and is usually the precursor stage of gum disease.
Signs and symptoms of gingivitis include:
• Red and swollen gum that might bleed during brushing
• Presence of receded gums or the elongated appearance of the teeth
• Bad breath or taste in the mouth
Gingivitis is easily reversible or treated with daily brushing and flossing as opposed to the severe irreversible damage caused by periodontitis. If gingivitis is not treated in time, it might evolve to the severe form, periodontitis. Periodontal diseases are made up of a group of infections which involves the supporting tissues of the teeth. The infections ranges in severity starting from mild to reversible inflammation of the gingiva to the destruction of periodontal tissues [2].
Signs and symptoms of periodontal disease include:
• Bright red, purple and swollen gums
• Receded gums or the elongated appearance of the teeth
• Bad breath or taste in the mouth
• Presence of pus between the teeth and gum
Untreated periodontitis might lead the irreversible destruction of the bones, gums and tissue supporting the teeth. In the more advance cases, the teeth might eventually become loose and had to be removed [8].
Application to biotechnology
Currently, there are studies performed to develop potential targets for the vaccine development against periodontitis using P. gingivalis antigenic determinants [9]. P. gingivalis virulence factors had been identified as potential targets for vaccines and currently, a DNA vaccine with a distinct potential for periodontal disease are currently undergoing phase I clinical trials [9].
Furthermore, research on the production of monoclonal antibodies against FimA protein of P. gingivalis were also performed [10]. As FimA protein is a crucial pathogenic component of the bacterium, complementary DNAs encoding for the heavy and light chains of the monoclonal antibody was cloned using a plant expression vector from Nicotiana benthamiana. In this research, the plant-derived monoclonal antibodies produced was able to bind specifically to the FimA protein purified from P. gingivalis. Thus, the result suggested that the newly created monoclonal antibody possess the ability to prevent P. gingivalis infection [10].
Current research
Porphyromonas gingivalis fimbriae: Recent developments describing the function and localization of mfa1 gene cluster proteins
As discussed earlier, P. gingivalis utilizes fimbriae to mediate initial host and pathogen interactions that are vital for the initiation and progression of the periodontal diseases [11]. P. gingivalis expresses two distinct fimbrial types, namely FimA and Mfa1. In type strain ATCC 33277, FimA fimbriae possess long filaments that are easily detachable from cells whereas Mfa1 fimbriae has easily detachable long filaments [11]. Both FimA and Mfa1 has five genes with the same transcriptional direction (fimA to fimE and mfa1 to mfa5).
Thus, in this study, a ATCC 33277-derived mfa2 mutant was discovered to produce long filaments that can easily detach from the cell surface. Thus, leading to the conclusion that Mfa2 also has a vital role in the anchoring and length regulation of Mfa1 fimbriae [11]. Additionally, a nonsense mutation in the fimB gene results in long FimA fimbriae.
Porphyromonas gingivalis downregulates the immune response of fibroblasts
P. gingivalis is one of the key pathogens in periodontitis [12]. There has been increasing evidence that periodontitis has association with systemic diseases. The anaerobic bacterium has several pathogenic properties which aid in the enhancement of growth and survival such as fimbriae and gingipains. Gingipains has been linked to the establishment and growth of P. gingivalis. Results had shown that it is involved in the regulation of the host’s inflammatory responses [12]. As such, P. gingivalis was observed to have evolved some of its mechanisms to evade the host immune system by the invasion of host cells and intercepting the signaling pathways by utilizing cytokines and degradation of receptors.
Therefore, in this research, it was observed that a range of fibroblast-derived inflammatory mediators were inactivated by P. gingivalis as a result of gingipain proteolytic activities [12].
To conclude, all of these new discoveries aid in the increased understanding of the innate and acquired immunity interaction between the periodontal bacteria and the host. It allowed the creation of new strategies and cures for the prevention and treatment of periodontitis and other related chronic inflammatory diseases.
References
8. National Institute of Dental and Craniofacial Research
This page is written by Chen Yanwen for the MICR3004 course, Semester 2, 2016