Leucobacter: Difference between revisions
(Created page with "{{Uncurated}} =Classification= ==a. Higher order taxa== ''Bacteria; Actinobacteria; Actinobacteria; Micrococcales; Microbacteriaceae; Leucobacter'' [1]. ==b...") |
|||
| Line 99: | Line 99: | ||
<br><br> | <br><br> | ||
<br>Edited by | <br>Edited by Jessica Kaminski, Jake Salzman, Christopher Turlo, and Hannah Wolf, students of [mailto:jmtalbot@bu.edu Jennifer Talbot] for [http://www.bu.edu/academics/cas/courses/cas-bi-311/ BI 311 General Microbiology], 2016, [http://www.bu.edu/ Boston University]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Jennifer Talbot at Boston University]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Jennifer Talbot at Boston University]] | ||
Latest revision as of 15:57, 12 December 2016
Classification
a. Higher order taxa
Bacteria; Actinobacteria; Actinobacteria; Micrococcales; Microbacteriaceae; Leucobacter [1].
b. Species within Leucobacter
Leucobacter alluvii; Leucobacter aridicolis; Leucobacter chironomi; Leucobacter chromiireducens; Leucobacter chromiiresistens; Leucobacter iarius; Leucobacter komagatae; Leucobacter luti
Description and significance
The genus Leucobacter is comprised of a variety of Gram-positive, non-motile, irregular rod-shaped bacteria. The genus was first characterized based upon the discovery of the species L. komagatae, named for Kazuo Komagata, the Japanese microbiologist who was the first to recognize this species [2]. The genus is widely dispersed and persists in environments that are chromium rich, such as chromite ore disposal sites [3], chromium rich treatment plant sludge [4], chromium contaminated water [5], and chromium rich soils [6]. Leucobacter is not limited by the presence of chromium, and can grow freely in its absence. Leucobacter iarius, for example, was discovered when attempting to identify bacteria in the nematode Steinernema thermophilum [7]. Although there are no known human pathogens among the genus Leucobacter, the genus has been studied for its remarkable capabilities for reducing toxic chromium compounds in its environment into stable, less toxic forms [3].
Phylogenetic Placement
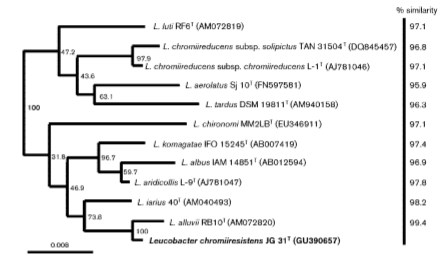
In 1996, when Leucobacter komagatae was first isolated, the 16S region of the ribosomal RNA was sequenced and grouped phylogenetically with microbes in the family Microbacteriaceae [2]. As more species of Leucobacter were isolated, the percentage of genetic similarity among Leucobacter species increased and allowed for the creation of phylotypes with high sequence similarity cutoffs. This prompted the diversification of species in the genus Leucobacter, as well as the identification of new strains within those species of Leucobacter. (Figure 2).
Genome structure
The G+C content of the genome is approximately 67% [4]. Ribosomal DNA was utilized to define most of the new species within the Leucobacter genus. Recently, DNA from one of the subspecies of Leucobacter was found in a metagenomic analysis of the microbiome of nematode species [9]. Detailed genomic analysis for this pathogenic form of Leucobacter (Leucobacter sp. AEAR) was performed to determine what genes were conserved in Leucobacter living within the host [9]. Of 2826 genes found, 2778 produced a protein product [9]. Confirmation was obtained through study of 123 consensus genes for Actinobacteria, a larger group encompassing the Leucobacter genus. Of the 123 observable genes tested only 8 were not found [9]. Three of those genes were involved in purine biosynthesis, a process that has been consistently absent among this subspecies [9]. The function of 738 of the 2778 protein encoding genes was undetermined, while 933 of the remaining protein encoding genes were responsible for biochemical processes [9]. Surprisingly, no genes involved in motility or chemotaxis were found to be present, and the amount of genes responsible for carbohydrate metabolism and cell signaling were depleted [9]. This gene loss is likely due to the inhabitation of the Leucobacter within the host, which can perform these processes and deliver nutrients to the Leucobacter in alternative methods [9].
Cell structure
Members of the genus Leucobacter are Gram-positive, rod-shaped organisms [2]. Colonies are circular, smooth, and opaque. Colonies are variant in color; however, most are white, yellow, or whitish-brown when grown on PY-BHI media plates [4]. Bacterial cells are 0.2-0.3 micrometers wide and 1.0-1.5 micrometers long [2]. Leucobacter are nonsporulating and do not produce mycelium [2]. L-diaminobutyric acid, alanine, glycine, and glutamic acid are present in the cell wall. Menaquinone 11, or MK-11, is the dominant respiratory quinone present in the cell wall [4]. Lawns of Leucobacter species exhibit biofilm-like characteristics, in that they were hydrophobic, sticky, and resistant to shear force [10].Leucobacter biofilms can inhibit the gene expression of infection-related genes in the nematode, Caenorhabditis elegans, leading to a reduced ability to fight infection and an increased mortality rate in the nematode [10].
Metabolic processes
Members of the genus Leucobacter are aerobic organisms, and therefore cannot ferment glucose [2]. Catalase and urease are produced; however, Leucobacter has no oxidase activity [2]. Members of the genus Leucobacter are known to persist at temperatures ranging from 15oC to 42oC, but grow optimally at 30oC [3]. Leucobacter can grow in conditions of pH 5-10; however, growth is optimal between pH 7-8 [5]. Leucobacter can also grow in concentrations of NaCl from 0-8% with a reduction of growth above 2% NaCl [8]. Members of this genus are highly chromate resistant, and can tolerate up to 300 mM K2CrO4 [4]. Nitrates are not used as a reducing agent in the cell. Leucobacter can hydrolyze Tween 40 and Tween 80. Gelatin and esculin are not hydrolyzed by the cell [2].
Ecology
Leucobacter is commonly found in environments that have been contaminated with chromium by industry, including aquatic areas, sludge, and soil samples [4][8. One species, Leucobacter sp. CRB1, is an aquatic species known to thrive in high concentrations of Cr(VI) and demonstrates the capability to reduce Cr(VI) in concentrations as high as 4000 mg/L [3]. Four other Leucobacter species - L. chromiireducens, L. aridicollis, L. luti, and L. alluvii - thrive in activated treatment plant sludge enriched with chromium [4][5]. Leucobacter are capable of living in alkaline environments due to the presence of industrial plant byproducts that raise the pH of the surrounding environment. For all species in this genus, growth only occurs in environments with low NaCl concentrations. There are no known attempts to combat Leucobacter with antibiotics as its pathogenic capabilities have only recently been discovered in a nonmammalian species, C. Elegans [10].
Pathology
No current research suggests that members of the Leucobacter genus are pathogenic to humans; however, research has shown a host-pathogen relationship between members of the genus Leucobacter and the nematode Caenorhabditis elegans [10]. In the presence of one strain of Leucobacter chromiireducens subsp. solipictus, there was increased host mortality rate, due to the development of cyst-like structures because of uterine infection [10]. The tested strain forms a hard, gel-like film in the uterine wall within 24 hours after inoculation and alters the change in expression of immunity-related genes, thus decreasing the nematode’s likelihood of fighting the infection [10].
Current Research
a. Host Bacterial Relationship with Caenorhabditis elegans

While generally identified as a chromium reducer, certain subspecies of Leucobacter have the potential for pathogenic interactions with nematodes and have proven pathogenic in the model nematode, C. elegans [10]. One such subspecies, Leucobacter solipictus strain TAN 31504, develops lawns in the uterus of the nematode C. elegans, resulting in the development of yellow cysts. Although it is not preferable, C. elegans can utilize Leucobacter solipictus strain TAN 31504 as a food source. C. elegans that are reared on TAN 31504 as a primary food source develop into thin adults with few fat stores. This process results in death after limited periods of exposure [10]. Infection can occur after 4 hours of exposure and persistently occurs if the incubation with TAN 31504 is longer than 12 hours [10]. TAN 31504 impacts expression of immunity-related genes in C. elegans. The TAN 31504 strain increases expression of the nlp-29 gene, which encodes an antimicrobial neuropeptide, previously known to be infection regulated [10]. TAN 31504 also forms a distinct, sticky and hydrophobic biofilm that is resistant to stress [10]. This biofilm formation can result in the formation of a swollen tail region due to the sticky extracellular matrix at the rectal end of the nematode [10]. In a recent study, two other strains of Leucobacter were responsible for forming a sticky extracellular matrix sugars, resulting in the formation of the worm-star, a lethal multi-nematode complex (Figure 3). The pathogenesis of Leucobacter in nematodes is currently being studied further and is used as a model for host-pathogen interactions through the formation of a biofilm [12].
b. Chromium Reduction Capabilities
What makes this genus markedly different from any other microbial genera is its ability to reduce hexavalent chromium [Cr(VI)] to the less toxic and less soluble trivalent chromium [Cr(III)] [4]. Leucobacter chromiiresistens and Leucobacter aridicollis both displayed growth in mediums with 5.0 mM Cr(VI) [4]. Though this test is a trademark for this genus, it is notable that chromium reduction occurs at different rates. These rates are related to the concentrations of the environments from which the bacterial strains were isolated and the composition of the peptidoglycan layer which differs slightly between Leucobacter species [4] [5]. This chromium reduction capability could potentially be utilized to help reverse chromium contaminated environments [3].Leucobacter sp. CRB1 is a chromium reducer of toxic Cr(VI) to Cr(III), which has a reduced toxicity and solubility which researchers sought to further understand. This strain can reduce chromium with a 100% efficiency in a concentration of 1000 mg/L and anaerobic conditions [3]. In comparison to other chromium reducers, Leucobacter sp. CRB1 has a higher reduction capability, with a maximum capacity of the strain at 2,490 mg/L [3].
References
[1]NCBI Taxonomy Browser. Leucobacter Taxonomy. Date Retrieved: 10/24/2016.
Edited by Jessica Kaminski, Jake Salzman, Christopher Turlo, and Hannah Wolf, students of Jennifer Talbot for BI 311 General Microbiology, 2016, Boston University.