Overview of Rhizopus oryzae: Difference between revisions
| Line 24: | Line 24: | ||
Include this section if your Wiki page focuses on a microbial process, rather than a specific taxon/group of organisms | Include this section if your Wiki page focuses on a microbial process, rather than a specific taxon/group of organisms | ||
=8. Current Research= | =8. Current Research= | ||
R. oryzae can cause postharvest disease in certain fruits such as apples and bananas [18]. This disease originates from soft rot, a fungal infection that occurs when products are stored at nonoptimal temperatures or extended periods of time at cold temperatures. When such fruits rot, white and cottony R. oryzae colonies will form on their surfaces [18]. | R. oryzae can cause postharvest disease in certain fruits such as apples and bananas [18]. This disease originates from soft rot, a fungal infection that occurs when products are stored at nonoptimal temperatures or extended periods of time at cold temperatures. When such fruits rot, white and cottony R. oryzae colonies will form on their surfaces [18]. | ||
Revision as of 21:52, 11 December 2017
1. Classification
a. Higher order taxa
Domain: Eukarya, Kingdom: Fungi, Phylum: Zygomycota, Class: Zygomycetes, Order: Mucorales, Family: Mucoraceae, Genus: Rhizopus, and Species: oryzae.
2. Description and significance
Describe the appearance, habitat, etc. of the organism, and why you think it is important.
- Include as many headings as are relevant to your microbe. Consider using the headings below, as they will allow readers to quickly locate specific information of major interest*
3. Genome structure
The genome of R. oryzae strain 99-880, which measures 45.3 Mb in length, contains numerous transposable elements (TEs) and coding sequences [3]. These TEs and coding sequences comprise 20% and 39% of the genome, respectively. The TEs of R. oryzae can be categorized into two classes: Class I and Class II. Class I TEs contain retrotransposons, while Class II TEs include DNA TEs [3]. In addition to TEs and coding sequences, the genome of R. oryzae contains expanded gene families involved in several processes such as cell wall synthesis, protein hydrolysis, and cell signaling [3]. The presence of the protease gene family in particular indicates a greater ability of R. oryzae to degrade organic matter. Additionally, the increase of the CHS and CDA gene families to 23 and 34 genes, respectively, accounts for the increase in chitin and chitosan within the microbe’s cell wall [3]. Other gene families found in R. oryzae transcribe proteins such as GTPases, GTPase regulators, chitin synthases (CHS), chitin deacetylases (CDA), secreted aspartic proteases, and subtilases [3]. Compared to other fungal microorganisms, R. oryzae possesses a highly repetitive genetic structure, as it contains ancestral genes similar to those contained in metazoan genomes rather than dikaryotic fungi genomes [3]. Such findings suggest that the genomic structure of R. oryzae observed today occurred as a result of whole genome duplication and subsequent sequence deletions [3].
4. Cell structure
R. oryzae grows as a result of mycelium accumulation [4]. Such accumulations are composed of tubular filaments known as hyphae. Mycelial cell walls, located in the body of the microbe, are reinforced by polysaccharides such as chitin [4]. Chitin reinforces the hyphae, creates a stronger cell wall, and thus allows for the cell to withstand greater pressures. While chitin provides a source of external, structural support to this microbe’s cells, lipids provide a source of internal, metabolic support as they can act as cellular storage sites [4].
5. Metabolic processes
Describe important sources of energy, electrons, and carbon (i.e. trophy) for the organism/organisms you are focusing on, as well as important molecules it/they synthesize(s).
6. Ecology
Habitat; symbiosis; contributions to the environment.
7. Pathology
How does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
7. Key microorganisms
Include this section if your Wiki page focuses on a microbial process, rather than a specific taxon/group of organisms
8. Current Research
R. oryzae can cause postharvest disease in certain fruits such as apples and bananas [18]. This disease originates from soft rot, a fungal infection that occurs when products are stored at nonoptimal temperatures or extended periods of time at cold temperatures. When such fruits rot, white and cottony R. oryzae colonies will form on their surfaces [18].
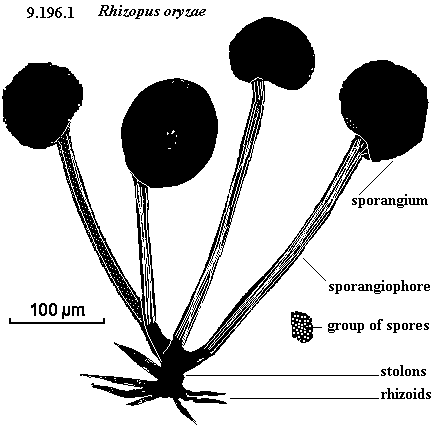
Visible structure of R. oryzae – sporangium is typically visible on decaying food as fuzz. http://www.uq.edu.au/_School_Science_Lessons/9.196.1.GIF
Although R. oryzae is a pathogenic microbe, it can be useful in food production [19]. Commercial R. oryzae cultures can be cooked and used as dry starters to inoculate different fruits and grains. As a fermentative agent, this microbe can enhance sweetness and acidity while producing a white, protective covering on food surfaces [19]. Although such food covers are edible, further research is needed to determine whether these products are safe to incorporate into the food industry completely. The new R. oryzae strain FSIS4 also produces an 𝛼-amylase enzyme that increases the volume and height-to-width ratio of bread [20]. Once purified via a three-phase partitioning technique, the enzyme is added to wheat flour at a concentration of 1.936 U per kg of flour. The increase in volume and height-to-width ratio may occur due to a reduced viscosity of the dough during starch gelatinization [20]. An aerated bread structure also results from the presence of 𝛼-amylase produced by the microbe. Bread utilizing commercial 𝛼-amylase creates a similar aerated structure, however with larger holes [20]. Upon further research, the effects of R. oryzae FSIS4 𝛼-amylase may prove useful in other food products as well.
9. References
1. Bala, K., Chander, J., Handa, U., Punia, R. S., and Attri, A. K. 2015. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Medical Mycology 53(3): 248–257.
2. Gryganskyi, A. P., Lee, S. C., Litvintseva, A. P., Smith, M. E., Bonito, G., Porter, T. M., Anishchenko, I. M., Heitman, J., and Vilgalys, R. 2010. Structure, function, and phylogeny of the mating locus in the Rhizopus oryzae complex. PLoS One 5(12):15-273.
3. Ma, L., Ibrahim, A. S., Skory, C., Grabherr, M. G., Burger, G., Butler, M., Elias, M., Idnurm, A., Lang, B. F., Sone, T., et al. 2009. Genomic Analysis of the Basal Lineage Fungus Rhizopus oryzae Reveals a Whole-Genome Duplication. PLOS Genetics 5(7):e1000549.
4. Mélida, H., Sain, D., Stajich, J. E., and Bulone, V. 2015. Deciphering the uniqueness of Mucoromycotina cell walls by combining biochemical and phylogenomic approaches. Environmental Microbiology 17:1649-1662.
5. Yin, X., Li, J., Shin, H., Du, G., Liu, L., and Chen, J. 2015. Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms: Advances and prospects. Biotechnology Advances 33(6):830-841.
6. Liu, Y., Xu, Q., Lv, C., Yan, C., Li, S., Jiang, L., Huang, H., and Ouyang, P. 2015. Study of metabolic profile of Rhizopus oryzae to enhance fumaric acid production under low pH condition. Applied Biochemistry and Biotechnology 177(7):1508-1519.
7. Londoño-Hernández, L., Ramírez-Toro, C., Ruiz, H. A., Ascacio-Valdés, J. A., Aguilar-Gonzalez, M. A., Rodríguez-Herrera, R., and Aguilar C. N. 2017. Rhizopus oryzae–Ancient microbial resource with importance in modern food industry. International Journal of Food Microbiology 257:110-127.
8. Hunter, W. E., Duniway, J. M., and Butler, E. E. 1977. Influence of nutrition, temperature, moisture, and gas composition on parasitism of Rhizopus oryzae by Syncephalis californica. Phytopathology 67:664-69.
9. Lengerova, M., Racil, Z., Hrncirova, K., Kocmanova, I., Volfova, P., Ricna, D., Bejdak, P., Moulis, M., Pavlovsky, Z., Weinbergerova, B., et al. 2014. Rapid detection and identification of mucormycetes in bronchoalveolar lavage samples from immunocompromised patients with pulmonary infiltrates by use of high-resolution melt analysis. Journal of Clinical Microbiology 52(8):2824-2828.
10. Dolatabadi, S., Kolecka, A., Versteeg, M., de Hoog, S. G., and Boekhout, T. 2015. Differentiation of clinically relevant Mucorales Rhizopus microsporus and R. arrhizus by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). Journal of Medical Microbiology 64:694-701.
11. Ibrahim, A. S., Spellberg, B., Avanessian, V., Fu, Y., and Edwards, J. E., Jr. 2005. Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infection and Immunity 73(2):778-783.
12. Chowdhary, A., Kathuria, S., Singh, P. K., Sharma, B., Dolatabadi, S., Hagen, F. and Meis, J. F. 2014. Molecular characterization and in vitro antifungal susceptibility of 80 clinical isolates of mucormycetes in Delhi, India. Mycoses 57(3): 97–107.
13. Shirazi, F., Kontoyiannis, D. P., and Ibrahim, A. S. 2015. Iron starvation induces apoptosis in Rhizopus oryzae in vitro. Virulence 6(2):121–126.
14. Mantadakis, E., and Samonis, G. 2009. Clinical presentation of zygomycosis. Clinical Microbiology and Infection 15(5):15-20.
15. Roden, M. M., Zaoutis, T. E., Buchanan, W. L., Knudsen, T. A., Sarkisova, T. A., Schaufele, R. L., Sein, M., Sein, T., Chiou, C. C., Chu, J. H., et al. 2005. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clinical Infectious Diseases 41(5):634-653.
16. Manesh, A., John, A. O., Mathew, B., Varghese, L., Rupa, V., Zachariah, A., and Varghese, G. M. 2016. Posaconazole: an emerging therapeutic option for invasive rhino-orbito-cerebral mucormycosis. Mycoses 59(12):765–772.
17. Tang, X., Zhu, H., Sun, L., Hou, W., Cai, S., Zhang, R., and Liu, F. 2014. Enhanced antifungal effects of amphotericin B-TPGS-b-(PCL-ran-PGA) nanoparticles in vitro and in vivo. International Journal of Nanomedicine 9:5403–5413.
18. Kwon, J., Ryu, J., Chi, T. T., Shen, S., and Choi, O. 2012. Soft rot of Rhizopus oryzae as a postharvest pathogen of banana fruit in Korea. Mycobiology 40(3):214-216.
19. Cantabrana, I., Perise, R., and Hernández, I. 2015. Uses of Rhizopus oryzae in the kitchen. International Journal of Gastronomy and Food Science 2(2):103–111.
20. Ait Kaki El-Hadef El-Okki, A., Gagaoua, M., Bourekoua, H., Hafid, K., Bennamoun, L., Djekrif-Dakhmouche, S., El-Hadef El-Okki, M. E., and Meraihi, Z. 2017. Improving bread quality with the application of a newly purified thermostable α-amylase from Rhizopus oryzae FSIS4. Foods 6(1).