Bacteria and Heart Disease: Difference between revisions
| Line 4: | Line 4: | ||
Physiologically, the heart is a synchronous ballet of various different muscles at work in order to supply the body’s cells with oxygen so that respiration can properly occur. There are a number of various things that can hinder this pumping of blood. One way would be the formation of a clot, due to high cholesterol diet or a low level of exercise. Another way, that isn’t so inconceivable would be the formation of a bacterial plague on the inner walls of the heart. Typical blood PH is buffered to around 6.7-7 (Cite). Furthermore, plenty of oxygen can be supplied to a bacterial colony (although they don’t necessarily need it) for respiration to occur. Another added benefit for a bacterial infection of the heart would be that any waste will get filtered and ultimately excreted by the host. So this leaves two questions unanswered: How does a bacterial infection occur in an organism? And, what types of microbial life can grow within a host heart? | Physiologically, the heart is a synchronous ballet of various different muscles at work in order to supply the body’s cells with oxygen so that respiration can properly occur. There are a number of various things that can hinder this pumping of blood. One way would be the formation of a clot, due to high cholesterol diet or a low level of exercise. Another way, that isn’t so inconceivable would be the formation of a bacterial plague on the inner walls of the heart. Typical blood PH is buffered to around 6.7-7 (Cite). Furthermore, plenty of oxygen can be supplied to a bacterial colony (although they don’t necessarily need it) for respiration to occur. Another added benefit for a bacterial infection of the heart would be that any waste will get filtered and ultimately excreted by the host. So this leaves two questions unanswered: How does a bacterial infection occur in an organism? And, what types of microbial life can grow within a host heart? | ||
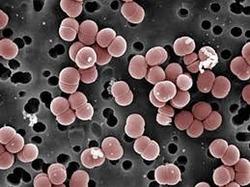
[[File:Enterococcus-faecalis.png|thumb|300px|right|SEM image of Enterococcus faecalis. Image credit: Janice Haney Carr, CDC/Pete Wardell. <ref>[https://www.medicalnewstoday.com/articles/318337.php<i>Leonard, Jayne. | [[File:Enterococcus-faecalis.png|thumb|300px|right|Figure 1: SEM image of Enterococcus faecalis. Image credit: Janice Haney Carr, CDC/Pete Wardell. <ref>[https://www.medicalnewstoday.com/articles/318337.php<i>Leonard, Jayne. “Enterococcus Faecalis: Infections, Transmission, and Treatment.” Medical News Today, MediLexicon International, www.medicalnewstoday.com/articles/318337.php.</i>]</ref> ]] | ||
[[File:E faecium vs E faecalis.jpg|thumb|300px|right|Figure 2: Agar plate colonies of A. Enterococcus faecium. B. Enterococcus faecali. C. both colonies on the same plate. <ref>[http://jcm.asm.org/content/48/3/999.full<i>Kallstrom, G., Doern, C. D., & Dunne, W. M. (2010). Evaluation of a chromogenic agar under development to screen for VRE colonization. Journal of Clinical Microbiology, 48(3), 999–1001. https://doi.org/10.1128/JCM.02011-09</i>]</ref> ]] | [[File:E faecium vs E faecalis.jpg|thumb|300px|right|Figure 2: Agar plate colonies of A. Enterococcus faecium. B. Enterococcus faecali. C. both colonies on the same plate. <ref>[http://jcm.asm.org/content/48/3/999.full<i>Kallstrom, G., Doern, C. D., & Dunne, W. M. (2010). Evaluation of a chromogenic agar under development to screen for VRE colonization. Journal of Clinical Microbiology, 48(3), 999–1001. https://doi.org/10.1128/JCM.02011-09</i>]</ref> ]] | ||
Revision as of 02:02, 11 May 2018
Introduction
Heart disease is the leading cause of death among both men and women killing an average of 610,000 people every year.[1] Furthermore, new research suggests that it is not just caused by high levels of cholesterol in the body. Instead, our hygiene has an important role to play in whether or not we get heart disease. Specifically, researchers at the University of Queensland in Australia suggest that our microbial flora has just as much to do with our risk of heart disease as our diet does. So, what role does the human microbiome play in our risk of heart disease? More importantly, how does the human microbiome contribute to heart disease? The researchers suggest that cardiovascular disease (CVD) can be caused by periodontal disease-causing bacteria via their transport from the gum tissues other regions in the body such as the heart, all the while retaining their pathogenicity.2 Furthermore, this pathogenicity triggers an immune response that leads to platlet formation in the arteries near the heart leading to CVD and eventually heart failure if left untreated.[2] Moreover, a number of potential factors can lead to various different forms of heart disease, for example: Environmental risk, genetic risk, diet, lifestyle and even including less objective measures like stress. The heart is one of the most complicated organs human beings have and understanding what can harm it allows researchers to understand how to prevent the various different types of heart disease.
Physiologically, the heart is a synchronous ballet of various different muscles at work in order to supply the body’s cells with oxygen so that respiration can properly occur. There are a number of various things that can hinder this pumping of blood. One way would be the formation of a clot, due to high cholesterol diet or a low level of exercise. Another way, that isn’t so inconceivable would be the formation of a bacterial plague on the inner walls of the heart. Typical blood PH is buffered to around 6.7-7 (Cite). Furthermore, plenty of oxygen can be supplied to a bacterial colony (although they don’t necessarily need it) for respiration to occur. Another added benefit for a bacterial infection of the heart would be that any waste will get filtered and ultimately excreted by the host. So this leaves two questions unanswered: How does a bacterial infection occur in an organism? And, what types of microbial life can grow within a host heart?
how Does infection Occur?
Specifically, Bacterial endocarditis (BE) or infective endocarditis (IE) is classified by an
infection of the inner lining (endocardia) by bacterial pathogens [6]. Typically, these bacteria are introduced into the bloodstream and settle around the values of the heart. Where do these bacteria come from, what types of bacteria are they, and how do they get into the blood stream? In order to address these questions, first we must establish a set of virulence factors (VFs) that allow for a disease to progress from benign to pathological. These factors specifically are associated with the pathogen Enterococcus faecalis (E. faecalis) when it infects a host organism. However, another main cause of BE is Enterococcus faecium (E. faecium).
Common Causes
The common causes of bacterial endocarditis include streptococcus, staphylococcus and
enterococcus [7]. All of these pathogens are commonly found around medical hospitals and can be common with post surgical infections. Furthermore, all of these diseases are treated via
antibiotics. However, certain strains are rapidly becoming resistant due to our misuse of antibiotic drugs. Two of these virulent strains E. faecalis and E. faecium help to illustrate the specific phenotypes required for thriving within the heart chambers. From adhering there in the first place to growing within the environment. Finally, with E. faecium, a specific example of these bacterial endocarditis infections.
Enterococcus faecalis
Classification
Higher order taxa Domain: Bacteria
Phylum: Firmicute
Class: Bacilli
Order: Enterococcaceae
Family: Enterococcus
Species: Enterococcus faecalis
Research
Aggregation substance (AS) protein is found in 30-40% of all BE strains of E. faecalis and is responsible for adhering the bacteria onto the endocardial surface [8]. Hemolysin is the next virulence factor being encoded on the same plasmid as the AS proteins. Furthermore, researchers showed a 55% mortality among Hemolysin+ AS+ strains while subsequently showing that Hemolysin+ AS- had no impact on mortality, nor did Hemolysin- AS+ to a significant degree[9]. This shows that both of these factors must be in place in order to cause pathogenic growth.
The next factor is cell wall glycolipids (CWG), and their role for causing biofilm formation. Researchers have shown that these E. faecalis glycolipids are an important part in causing BE pathogenicity. Specifically, two glucosyltranferases (A & B) are required for the biosynthesis of the two main cell wall glycolipids (diglycosyl-diacylglycerol & monoglycosyl- diacylglycerol) [9]. Researchers have also shown that a general stress protein is important for virulence of the E. faecalis BE strain [9]. Essentially, these two factors are able to keep the pathogen alive for when conditions return to normal when not in the heart.
Current research has also looked at the role of Biofilm-Associated Pili (Ebp) on virulence. These pili are able to adhere the bacteria to the surface of the endocardia and promote the growth of the strain by allowing biofilm to grow. This is very similar to the AS protein but is primarily focused on the pili instead of the surface protein. The next factor is Gelatinase (GelE) which allows the bacteria to breakdown host collagen, fibrinogen and fibrin. This allows the pathogen E. faecalisto obtain carbon sources other than glucose from the host. Furthermore, there is no competition for these carbon sources because human cells don’t have this protein.
Adhesin to Collagen of E. Faecalis (Ace) is the next E. faecalisvirulence factor and its role is to primarily facilitate the anchoring of the E. faecalis to collagen like that found in the heart values. Likewise, Enterococcal Fibronectin-Binding Protein A (EfbA) I another adhesin protein that helps the adhesion to immobilized fibronectin, like that found in cardiac endothelium. And finally, the Eep metalloprotease promotes the processing of sex pheromone peptides that induce the conjugation of large pheromone response plasmids, like the AS protein. The authors
of this review on the E. faecalis virulence then rated the contribution of each factor by the involvement of the factor in the virulence of the strain.
Their conclusion was that factors that allowed the bacteria to adhere to the surface were the most important to conferring virulence. This makes sense because if the bacteria cannot adhere to the heart than they cannot form a proper biofilm and cannot grow. Furthermore, the heart pumps blood throughout the body at an incredible rate and bacteria will have to attach tightly to the endocardia in order to facilitate growth. The less important factors included Hemolysin and Gls24, which both are important in conjunction with other factors. However, on their own they confer no virulence. The other major cause of BE is E. faecium.
Enterococcus faecium
Classification
Higher order taxa
Domain: Bacteria
Phylum: Firmicute
Class: Bacilli
Order: Enterococcaceae Family: Enterococcus
Species: Enterococcus faecium
Research
Medical researchers presented a case of a 71-year-old woman who developed an infection of E. faecium after receiving heart surgery for treatment of tricuspid and mitral valve regurgitation. After growing blood cultures, the researchers came to the conclusion that E. faecium was the confirmed infection that persisted in the woman. This confirmation was a result of the Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) analysis of the bacteria grown on the culture. Furthermore, the researchers showed that this strain of E. faecium was classified as a small colony variant (SCV) which are slow growing and hard to detect without MALDI-TOF analysis. Finally, this strain, in particular, was susceptible to gentamicin (antibiotic, figure X). However, more and more antibiotic resistant strains of BE causing bacteria are emerging as a result of our overuse of the drugs. This medical case study starts to hint at possible paths to infection that these bacteria can take to get into the bloodstream. The first of which is through surgery and the second could be through drug use in our ever-expanding opioid epidemic.
Metabolism of Enterococcus
The various species of the Enterococci family have similar metabolisms to the members of the firimicutes (like clostridia). Interesting metabolisms that E faecalis and E. faecium have making them thrive within the bloodstream include catabolizing inositol, which is a carbon source that is not found in other strains of Enterococci [9]. In humans this carbon source can be biosynthesized by the kidneys as a byproduct in glycolysis [10]. This, has implications for the bacteria being able to thrive in the blood, because if they can catabolize a sugar made in the kidneys than the bacteria are capable of surviving in the blood stream.
Surgery
Any time someone gets surgery to treat a problem, there are certain risks that person is taking. Firstly, any time a person goes into surgery, bacteria can be on the skin that, once an incision is made, can get into the body. During open heart surgery, this is a big problem because doctors have to cool the heart down in order to slow its beating and perform the operation. Blood flow greatly decreases during this time and it gives bacteria a chance to enter the body through the capillaries. When the surgery is done and over with, blood flow is restored and the newly introduced pathogens are free floating throughout the body.
Drug Use
As the opioid epidemic rages on in America, more and more people are turning to a cheap supply of needle-based narcotics to get high. Unsurprisingly, diseases that are contracted from “dirty needles”, or Skin and soft tissue infections (SSTIs) are a problem for the people who use illegal drugs do the unsafe practices for getting high. Needles in particular, create problems for contracting blood borne disease such as the ones described above. E. faecalis and E. faecium pose significant problems for these people because the pathogen can hitch a direct ride to the bloodstream. In order to help combat these problems many social programs, including needle exchange programs are being funded for the most affected areas [11]. Furthermore, research has been conducted on drug abuser hospitalizations by the CDC. They found that drug-dependence associated endocarditis has risen eighteenfold between 2010-2015, from $1.1 million to $22.2 million [12]. Furthermore, the CDC found that the people most affected by this are aged between 18 and 40 [12]. As the CDC reported, the opioid epidemic has had a large effect on the US population, specifically on the healthcare system. Not only do healthcare workers have to deal with the overdose effects of illegal drugs, they also have to worry about potential heart infections that could have adverse effects on the heart of a patient.
Oral Hygeine
Another less serious method of bacteria entering the bloodstream is through our mouths. When we brush our teeth or chew gum, we can cause micro-cuts that allow bacteria to enter the blood. Most microbes will be dealt with by the immune system. However, in people with weakened immune systems, or who already have an infection, certain microbes can enter the cardiovascular system and infect the heart leading to BE [13]. researchers that performed a study on oral-originating endocarditis(OOE) have shown that the primary cause of OOE are bad hygiene patterns and whether or not the person has had a dental procedure in the last 3 months [14]. This finding suggests that keeping a proper oral hygiene prevents someone from contracting endocarditis. However, as the saying goes, too much of a good thing can also be bad because if bacteria exists on a toothbrush or dental floss than it can enter the bloodstream via microtears in the gums.
Link between oral hygiene and endocarditis
Poor oral health has been shown to be a risk factor in patients that have been treated for BE [15]. Furthermore, poor oral health leads to the inflammation of the gums (gingivitis), which will subsequently lead to microbes entering the bloodstream via the mouth. Moreover, the mouth can be colonized by a vast array of microorganisms [16]. Some of these organisms include various BE causing pathogens like the ones described above. The researchers conclude that their study may not be a healthy representation of the broader adult population because they only used people that were in need of a tooth extraction in the first place. This made them predisposed to various bacterial diseases that other health people might not have [17]. Finally, the researchers make the argument that methods used to prevent BE infection should focus on good oral hygiene in order to reduce the number of bacteria entering the blood.
Future Research
This area is still an ever-expanding field, and rapid growth into the prevention of heart disease is being taken every year. Furthermore, studies that link other aspects of health like oral health, will help to establish more preventative ways for limiting heart disease. Moreover, understanding the risk factors of age on heart health. Endocarditis in particular, has been shown to affect primarily older people due to their weakened immune system [18]. However, what the relation is with younger age people who are hospitalized frequently, or younger aged people who don’t necessarily take care of their teeth.
Conclusion
Bacterial related heart disease has been shown to be a serious problem among a very diverse groups of people. Two bacterial species in particular, E. faecalis and E. faecium have been shown to be the major cause of bacterial Endocarditis (BE). Bacterial Endocarditis is a infection of the epithelial cells (endocardia), caused by the adhesion of enterococci bacteria to the mitral and tricuspid valves in the heart. The more extensively studied E. faecalis has been shown to have virulence factors that contribute to the pathogenicity. Furthermore, these infections are shown to be caused in a number of ways including illegal drug use, surgery, and more commonly poor oral hygiene.
References
- ↑ CDC Fact Sheet of Heart Disease
- ↑ Ford, J. P., Do, L. H., and Leishman, J. S.Cardiovascular disease and the role of oral bacteria. 2010. J Oral Microbiol.
- ↑ Leonard, Jayne. “Enterococcus Faecalis: Infections, Transmission, and Treatment.” Medical News Today, MediLexicon International, www.medicalnewstoday.com/articles/318337.php.
- ↑ Kallstrom, G., Doern, C. D., & Dunne, W. M. (2010). Evaluation of a chromogenic agar under development to screen for VRE colonization. Journal of Clinical Microbiology, 48(3), 999–1001. https://doi.org/10.1128/JCM.02011-09
- ↑ unique biotech limited
- ↑ art-Valves-and-Infective-Endocarditis_UCM_450448_Article.jsp#.Wt6HJtPwYWo Heart Valves and Infective Endocarditis. (2017). Retrieved April 23, 2018, from http://www.heart.org/HEARTORG/Conditions/More/HeartValveProblemsandDisease/He art-Valves-and-Infective-Endocarditis_UCM_450448_Article.jsp#.Wt6HJtPwYWo
- ↑ Mcdonald, J. R. (2009). Acute I nfe ctive Endoc arditis. Infectious Disease Clinics of NA, 23(3), 643–664.
- ↑ Madsen, K. T., Skov, M. N., Gill, S., & Kemp, M. (2017). Virulence Factors Associated with Infective Endocarditis: A Mini Review. The Open Microbiology Journal, 11(1), 1– 11.
- ↑ 3
- ↑ Parthasarathy, L. K., Seelan, R. S., Tobias, C., F, M., & Parthasarathy, R. N. (2006). Mammalian Inositol 3-phosphate Synthase : Its Role in the Biosynthesis of Brain Inositol and its Clinical Use as a Psychoactive Agent. In Biology of Inositols and Phosphoinositides (Vol. 39, pp. 293–314). .
- ↑ Irish, C., Maxwell, R., Dancox, M., Brown, P., Trotter, C., Verne, J., & Shaw, M. (2007). Skin and soft tissue infections and vascular disease among drug users, England. Emerging Infectious Diseases, 13(10), 1510–1511.
- ↑ Fleischauer, A. T., Ruhl, L., Rhea, S., & Barnes, E. (2017). Hospitalizations for Endocarditis and Associated Health Care Costs Among Persons with Diagnosed Drug Dependence — North Carolina, 2010–2015. MMWR. Morbidity and Mortality Weekly Report, 66(22), 569–573. https://doi.org/10.15585/mmwr.mm6622a1
- ↑ Lockhart, P. B., Brennan, M. T., Thornhill, M., Michalowicz, B. S., Noll, J., Bahrani- Mougeot, F. K., & Sasser, H. C. (2009). Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. Journal of the American Dental Association, 140(10), 1238–1244.
- ↑ Duval, X., Millot, S., Chirouze, C., Selton-Suty, C., Moby, V., Tattevin, P., … Alla, F. (2017). Oral streptococcal endocarditis, oral hygiene habits, and recent dental procedures: A case-control study. Clinical Infectious Diseases, 64(12), 1678–1685. https://doi.org/10.1093/cid/cix237
- ↑ 5
- ↑ 6
- ↑ 6
- ↑ Strom, B. L., Abrutyn, E., Berlin, J. A., Kinman, J. L., Feldman, R. S., Stolley, P. D., ... Kaye, D. (2000). Risk factors for infective endocarditis: Oral hygiene and nondental exposures. Circulation, 102(23), 2842–2848.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.