The Gut Microbiome and Anxiety: Difference between revisions
No edit summary |
No edit summary |
||
| Line 27: | Line 27: | ||
==Clinical Implications== | ==Clinical Implications== | ||
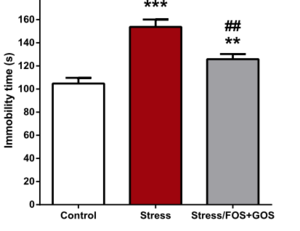
[[Image: BurokasFigure.png | thumb | | [[Image: BurokasFigure.png | thumb | 300px | right | Figure 4. The effects of FOS+GOS treatment on a forced swim test in chronically stressed mice. Burokas et al. 2017. <ref name=xx/>[https://www.sciencedirect.com/science/article/pii/S0006322317300422] ]] | ||
With the knowledge of which microbes may be important as well as how they may act upon mental health, the impact of the gut microbiome on anxiety has clinical and public health implications. There is strong evidence that microbes could be a viable treatment option for anxiety. <i>Lactobacillus</i>, in particular, has strong promise for acting as a pharmaceutical anxiolytic.<ref name=vv/> <i>L. plantarum</i> both significantly reduced anxiety-related behavior but, important for clinical use, had no effect on other major physiological processes and vital signs.<ref name=vv/> Encouragingly, treatment options may not be limited to ingesting a single microbe, or even to taking probiotics consisting of many microbes. There is evidence that prebiotics, compounds that induce growth and activity of microbes already present in the gut, are sufficient in helping our gut microbiomes help us. Fruto-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) are two prebiotics, and both together and GOS independently were successful as an antidepressant and anxiolytic in mice. <ref name=xx>[https://www.sciencedirect.com/science/article/pii/S0006322317300422=PDF Burokas, A., Arboleya, S., Moloney, R.D., Peterson, V.L., Murphy, K., Clarke, G., Stanton, C., Dinan, T.G., Cryan, J.F. “Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice.” 2017. Biological Psychiatry. 82:472-487.]</ref> In response to choric stress, these prebiotics lowered cortisone release, altered gene expression in the brain, and behaviorally reduced depression and anxiety-linked activity. For example, in a forced swim test, chronically stressed mice that had been treated with FOS and GOS exhibited less immobile time compared to their stressed counterparts (Figure 4). These findings are exciting; the gut microbiome is a promising avenue for new anxiolytic treatments. | With the knowledge of which microbes may be important as well as how they may act upon mental health, the impact of the gut microbiome on anxiety has clinical and public health implications. There is strong evidence that microbes could be a viable treatment option for anxiety. <i>Lactobacillus</i>, in particular, has strong promise for acting as a pharmaceutical anxiolytic.<ref name=vv/> <i>L. plantarum</i> both significantly reduced anxiety-related behavior but, important for clinical use, had no effect on other major physiological processes and vital signs.<ref name=vv/> Encouragingly, treatment options may not be limited to ingesting a single microbe, or even to taking probiotics consisting of many microbes. There is evidence that prebiotics, compounds that induce growth and activity of microbes already present in the gut, are sufficient in helping our gut microbiomes help us. Fruto-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) are two prebiotics, and both together and GOS independently were successful as an antidepressant and anxiolytic in mice. <ref name=xx>[https://www.sciencedirect.com/science/article/pii/S0006322317300422=PDF Burokas, A., Arboleya, S., Moloney, R.D., Peterson, V.L., Murphy, K., Clarke, G., Stanton, C., Dinan, T.G., Cryan, J.F. “Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice.” 2017. Biological Psychiatry. 82:472-487.]</ref> In response to choric stress, these prebiotics lowered cortisone release, altered gene expression in the brain, and behaviorally reduced depression and anxiety-linked activity. For example, in a forced swim test, chronically stressed mice that had been treated with FOS and GOS exhibited less immobile time compared to their stressed counterparts (Figure 4). These findings are exciting; the gut microbiome is a promising avenue for new anxiolytic treatments. | ||
<br> | <br> | ||
Revision as of 01:34, 4 April 2021
Introduction to the Gut-Brain Axis
By Laura Grosh
When people think of organs, they would likely not list the gut microbiome. Increasing research, however, is starting to change this assumption. The gut microbiome is a dynamic collection of microbes that live in our intestinal tract, and we are beginning to see that this microbial community is as integral to our health as the organs that may initially come to mind such as the heart or lungs. The gut microbiome is huge; recent estimates state that in a 70 kg person, 0.2 kg are bacteria, many of which reside in the gut. [1] One way that the gut microbiome is vital to us in through their connection to our nervous system. Microbes are directly responsible for making neurotransmitters and neuropeptides, integrating them into the nervous system—but the connection between these seemingly separated systems extends beyond this. The vagus nerve connects the brain and the gut, a direct representation of the gut-brain axis. [2] Autonomic, immune, and endocrine responses further complicate and interact with this connection between the gut microbiome and our nervous system. Research has addressed this connection by performing metagenomic analyses on the gut microbiome, studying model organism lacking commensal bacteria, altering the gut microbiome through probiotics or antibiotics, activating or deactivating the vagus nerve, among many other molecular and genomic manipulations [3] Studying this connection, as complicated as it is, is beginning to uncover the implications of the gut microbiome on mental health. The gut microbiome is increasingly believed to facilitate relationships between stress and anxiety, both in direct and indirect ways.[4] This has direct implications on public health and the field of mental health, and could even increase treatment options for anxiety in the future.
Stress and the HPA axis
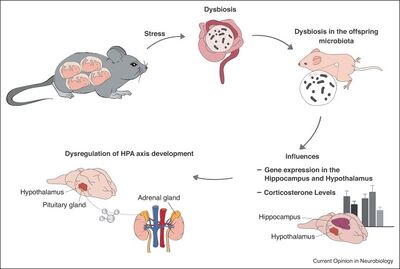
One way that the gut microbiome may influence anxiety is through the relationship between the gut microbiome and the HPA axis, stress and inflammation. The HPA axis is a system that modulates stress responses. When a physical or psychological stressor appears, the hypothalamus sends signals to the pituitary gland, which releases adrenocorticotropic hormone. This hormone acts on the adrenal glands to release cortisol, which is the bodies main stress hormone. In an ideal world, cortisone provides negative feedback for both the hypothalamus and pituitary gland (Figure 1). [6] Of course, in the less than perfect body’s we inhabit and in an increasingly stressful world, this can go sometimes go wrong. Our stress response is not the same thing as clinical anxiety, but there are many correlations that make the HPA axis an interesting thing to study when asking questions about anxiety. Dysregulation of the stress response and HPA axis, behaviorally observed as chronic stress, is highly co-morbid with anxiety. [7] To further support the connection between HPA axis and anxiety, patients with anxiety disorders show an increased cortisol response, suggesting an increase in HPA axis activity. [8] Understanding how the gut microbiome influences the HPA axis is the first step in understanding how the gut microbiome affects anxiety.
The influence of the microbiome on stress and the HPA axis may start from birth. Observed in mice, maternal stress during pregnancy and offspring stress in early life both lead to dysbiosis (an abnormal gut microbiome) and altered development of the HPA axis (Figure 1). [9] [5] This is likely because both the gut microbiome and the HPA axis are not yet developed at birth. These findings lead us to believe that the gut microbiome, both at birth and in early development, is connected to the development of the HPA axis. [10]
Germ free (GF) mice are a common model organism for studying the relationship between gut microbiota and behavior. GF mice are raised without exposure to microorganisms, giving us insight into what behaviors and processes are impacted without a microbiome. Using GF mice has proven to be extremely useful when studying the gut-brain axis. [11]
Multiple studies have used GF mice to examine HPA development and function. Fascinatingly, not all studies using GF mice yield the same effects on HPA function. One such study, done by Huo et al. in 2017, used a population of GF mice and mice free from specific pathogens (specific pathogen free mice, or SPF mice). After chronic restraint to induce stress, the researchers measured exploratory time, a behavior associated with stress level, as well as level of hormones released by the HPA axis. In the stressed subject groups, they found that GF mice had significantly higher stress hormone levels compared to SPF mice. However, the GF mice exhibited more exploratory behavior than the SPF mice. [12] Other studies, however, have found an increase in anxiety-associated behavior in GF rats compared to SPF rats, and similar dysregulation of HPA axis-related hormones. [13] Even though there is contradictory evidence whether GF animals experience more or less anxiety, it is clear that the microbiome does play a role in anxiety-like behavior by modulating the HPA axis.
Hormones, Neurotransmitters, and Peptides
To understand how the gut microbiome plays a role in anxiety, we must understand what hormones, neurotransmitters, and peptides are involved in mediating this relationship. By studying the HPA axis, we have established that the hormones in the HPA axis—corticotropin releasing hormone, adrenocorticotropic hormone, and cortisol—are important. While the HPA axis is involved in anxiety, we must also examine other neurotransmitters and peptides that may be more directly involved in anxiety.
Neurotransmitters are chemicals that act on neurons, but they are not limited to the central nervous system. In fact, many neurotransmitters are found in the gut, made by the gut microbiome. [15] Some examples of this include Candida or Echerichia synthesizing and releasing serotonin, or dopamine synthesized by Bacillus. [15] These are common gut microbes. While these neurotransmitters cannot always cross the blood-brain barrier, they can still act on parts of the peripheral nervous system and the vagus nerve. [15] Somewhat counterintuitively, peptides may be how the gut microbiome is involved in anxiety. The volume of gut peptides and neurotransmitters that the gut microbiome is responsible for in some part (synthesizing, stimulating release, altering, or activating) makes the gut one of the largest centers of endocrinology, immunology, and neuroscience. There are many types of peptides influenced by microbes, but neuropeptide Y (NPY), peptide YY (PYY), and pancreatic polypeptide (PP), are three peptides most commonly implicated in anxiety. [16]
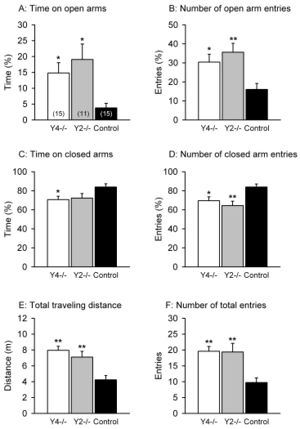
One study that showed the effects of NPY on anxiety was conducted by Painsipp et al. in 2008. [14] There are six classes of NPY receptors. This study used mice that either lacked the NPY2 receptor (Y2-/-) or NPY4 receptor (Y4-/-). Using a variety of anxiety-linked behavioral tests, they were able to compare these groups with a control group to elucidate the effects of NPY via these two receptors on anxiety. They found that the Y4-/- mice and Y2-/- mice had reduced anxiety-like behavior for behavioral tests such as the elevated plus maze (Figure 2), but only Y4-/- exhibited reduced anxiety-like behavior in other tests. [14] However, the control group never exhibited less anxiety-like behavior than either knockout. In an elevated plus maze, there is a plus-shaped maze elevated off the ground with two arms open and two arms enclosed. More time on the open arms as well as more exploratory behavior indicates a lower level of anxiety; an anxious mouse will spend more time in closed arms, taking cover. Reduced anxiety can be inferred through every measure of the elevated plus maze for the Y4 and Y2 knockouts. This helps us understand how the NPY2 and NPY4 receptors are important in anxiety. The gut microbiome and NPY both influence each other; this study more directly tying the gut microbiome to molecules that are important in anxiety. [17]
Microbial Friends and Foes
The gut microbiome can modulate anxiety through its influence on the HPA axis as well as through hormones, neurotransmitters, and peptides. But which microbes are important in this? There is evidence that the bacterial genus Lactobacillus is one such type of microbe that plays a role in anxiety. Many species of Lactobacillus are popular probiotics, conferring many health benefits. [18] Both Lactobacillus rhamnosus and Lactobacillus plantarum have been shown to decrease anxiety.
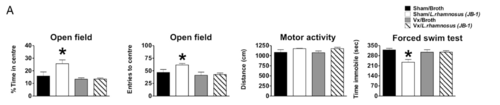
In a 2011 study, mice fed L. rhamnosus in the form of probiotics exhibited less anxiety-related behavior, lowered levels of cortisone, and differential GABA receptor expression. [19] Importantly, these results were not seen in mice whose vagus nerves were severed; showing both the effects of L. rhamnosus on anxiety, but also reinforcing the importance of the vagus nerve in how the gut microbiome communicates with the central nervous system. [19] These behavioral results can be seen in Figure 3. L. plantarum ingestion in GF mice similarly reduced anxiety. [20] Unlike L. rhamnosus, L. plantarum did not affect the HPA axis, but similar reduced anxiety-related behavior. It also increased brain concentrations of dopamine and serotonin.[19]
Not all bacteria have beneficial effects on their host’s health. Citrobacter rodentium is a pathogenic microbe of the gut microbiome. Infection of C. rodentium in mice increased anxiety-related behavior, seen through significantly decreased exploratory time in uncovered areas, decreased total distance explored, and displayed more risk assessing behavior. [21] Studies using specific gut microbes show how individual strains may affect anxiety; some increasing levels of anxiety and some decreasing levels of anxiety.
Clinical Implications
With the knowledge of which microbes may be important as well as how they may act upon mental health, the impact of the gut microbiome on anxiety has clinical and public health implications. There is strong evidence that microbes could be a viable treatment option for anxiety. Lactobacillus, in particular, has strong promise for acting as a pharmaceutical anxiolytic.[20] L. plantarum both significantly reduced anxiety-related behavior but, important for clinical use, had no effect on other major physiological processes and vital signs.[20] Encouragingly, treatment options may not be limited to ingesting a single microbe, or even to taking probiotics consisting of many microbes. There is evidence that prebiotics, compounds that induce growth and activity of microbes already present in the gut, are sufficient in helping our gut microbiomes help us. Fruto-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) are two prebiotics, and both together and GOS independently were successful as an antidepressant and anxiolytic in mice. [22] In response to choric stress, these prebiotics lowered cortisone release, altered gene expression in the brain, and behaviorally reduced depression and anxiety-linked activity. For example, in a forced swim test, chronically stressed mice that had been treated with FOS and GOS exhibited less immobile time compared to their stressed counterparts (Figure 4). These findings are exciting; the gut microbiome is a promising avenue for new anxiolytic treatments.
Finding treatments for mental health conditions like anxiety are important from a public health standpoint as well. Mental health is not only important in its own right, but is the locus for a major public health crisis and contains many health disparities and inequities. In 21 countries surveyed by the World Mental Health surveys, only 9.8% of people diagnosed with anxiety received adequate treatment, and lower income countries exhibited lower treatment levels. [23] Compared to their cisgender counterparts, transgender youth have significantly higher rates of anxiety.[24] Exposure to chronic stressors such as homelessnesses, abuse, and systemic violence have not only created disparities in anxiety prevalence, but socioeconomic status has created inequities in treatment of anxiety disorders. [25] We often think of infectious disease as a microbial based pathology that may result in health disparities, but the link between the gut microbiome and mental health makes disparities of mental health part of the microbial world as well. While these disparities are disheartening, microbial treatments for anxiety (some as simple as probiotics or prebiotics) may help to lessen these health disparities.
References
- ↑ Sender, R., Fuchs, S., Milo, R. “Revised “Estimates for the Number of Human and Bacteria Cells in the Body” 2016. PLoS Biology 14:8, 1002533.
- ↑ Browning, K., Verheijden, S., and Boeckxstaens, G."The Vagus Nerve in Appetite Regulation, Mood, and Intestinal Inflammation" 2017. Gastroenterology 152:4, 730-744.
- ↑ Foster, J. and McVey Neufeld, K.” Gut-brain axis: how the microbiome influences anxiety and depression.” 2013. Trends in Neurosciences: 36:5, 305-312.
- ↑ Peirce, J. and Alvina, K. "The role of inflammation and the gut microbiome in depression and anxiety" 2019. J. Neurosci. Res. 97:10, 1223-1241.
- ↑ 5.0 5.1 Fankiensztajn, L.M., Elliott, E., Koren, O. “The microbiota and the HPA axis, implications for anxiety and stress disorders.” 2020. Current Opinion in Neurobiology. 62:76-82.
- ↑ Godoy, L.D., Rossignoli, M.T., Delfino-Pereira, P., Garcia-Cairasco, N., de Lima Umeoka, E.H. “A comprehensive overview on stress neurobiology: Basic concepts and clinical implications” 2018. Front Behav Neurosci. 12:127.
- ↑ Fernandes, V., Osorio, F.L. “Are there associations between early emotional trauma and anxiety disorders? Evidence from a systematic literature review and meta-analysis.” 2015. Eur Psychiatry. 30:6, 756-765.
- ↑ Zorn, J.V., Schur, R.R., Boks, M.P., Kahn, R.S., Joels, M., Vinkers, C.H. “Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis.” 2016. Psyneuen. 77:25-36.
- ↑ Gur, T.L. Palkar, A.V. Rajesekera, T., Allen, J., Niraula, A., Godbout, J., Bailey, M.T. “Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring.” 2019. Behav Brain Research. 359:886-894.
- ↑ de Weerth, C. “Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis.” 2017. Neuro and Behav Reviews. 83:458-471.
- ↑ Luczyniski, P, Neufeld, K.M., Oriach, C.S., Slarke, G., Dinan, T.G., Cryan, J.F. “Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior” 2016. Int J Neuropsychopharmacol. 19:8.
- ↑ Huo, R., Zeng, B., Zeng, L., Cheng, K., Li, B., Luo, Y., Wang, H., Zhou, C., Fang, L., Li, W., Niu, R., Wei, H., Xie, P. “Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis.” 2017. Front. Cell. Infect. Microbio.
- ↑ Crumeyrolle-Arias, M., Jaglin, M., Bruneau, A., Vancassel, S., Dauge, V., Naudon, L., Rabot, S. “Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats.” 2014. Psychoneuroendocrinology. 42:207-217.
- ↑ 14.0 14.1 14.2 Painsipp, E., Wultsch, T., Edelsbrunner, M., Tasan, R., Singewald, N., Herzog, H., Holzer, P. “Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice” 2008. Genes Brain Behav. 7:5, 532-542.
- ↑ 15.0 15.1 15.2 Sun, L., Li, J., Nie, Z. “Gut hormones in microbiota-gut-brain cross-talk” 2020. Chinese Medical Journal 133:7, 826-833.
- ↑ Lach, G., Schellekens, H., Dinan, T.G., Cryan, J.F. “Anxiety, depression, and the microbiome: a role for gut peptides.” 2018. Neurotherapeutics. 15:1, 36-59.
- ↑ Holzer, P., Farzi, A. “Neuropeptides and the Microbiota-Gut-Brain Axis” 2014. Adv Exp Med Biol. 817:195-219.
- ↑ O’Callaghan, J., O’Toole, P. “Lactobacillus: Host-Microbe Relationships.” 2011. Between Pathogenicity and Commensalism pages 119-154.
- ↑ 19.0 19.1 19.2 19.3 Bravo, J.A., Forsythe, P., Chew, M.V., Escaravage, E., Savignac, H.M., Dinan, T.G., Beinenstock, J., Cryan, J.F. “Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in mouse via the vagus nerve.” 2011. PNAS 108:38, 16050-16055.
- ↑ 20.0 20.1 20.2 Liu, W., Chuang, H., Huang, Y., Wu, C., Chou, G., Wang, S., Tsai, Y. “Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice.” 2016. Behav Brain Res. 298:202-209.
- ↑ Lyte, M., Li, W., Opitz, N., Gaykema, R.P.A., Goehler, L.E. “Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium.” 2006. Phys and Behav. 89:350-357.
- ↑ 22.0 22.1 Burokas, A., Arboleya, S., Moloney, R.D., Peterson, V.L., Murphy, K., Clarke, G., Stanton, C., Dinan, T.G., Cryan, J.F. “Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice.” 2017. Biological Psychiatry. 82:472-487.
- ↑ Alonso, J. “Treatment gap for anxiety disorders is global: results of the World Mental Health Surveys in 21 countries.” 2018. Depression and Anxiety. 35:3, 195-208.
- ↑ Reisner, S.L., Vetters, R.V., Leclerc, M., Maslow, S., Wolfrum, S., Shumer, D., Mimiaga, M.J. “Mental health of transgender youth in care at an adolescent urban community health center: a matched retrospective cohort study.” J. Adolescent Health. 56:3, 274-279.
- ↑ Zvolensky, M.J., Gary, L., Bakhshaie, J. “Disparities in anxiety and its disorders.” 2017. J Anxiety Disorders. 48:1-5.