Vibrio cholerae: Difference between revisions
No edit summary |
No edit summary |
||
| Line 52: | Line 52: | ||
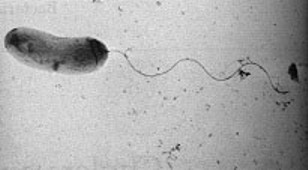
''V. cholerae'' is Gram-negative has what is called a comma shaped cell body and contains a singular polar flagellum used for motility.<sup>1</sup> ''V. cholerae'' serogroup O1 does not have a polysaccharide capsule, but serogroup O139 does contain a polysaccaride capsule made up of "N-acetylglucosamine, N-acetylquinovosamine (QuiNAc), galacturonic acid (GalA), and galactose and two residues of 3,6-dideoxyxylohexose (Xylhex)."<sup>16</sup> | ''V. cholerae'' is Gram-negative has what is called a comma shaped cell body and contains a singular polar flagellum used for motility.<sup>1</sup> ''V. cholerae'' serogroup O1 does not have a polysaccharide capsule, but serogroup O139 does contain a polysaccaride capsule made up of "N-acetylglucosamine, N-acetylquinovosamine (QuiNAc), galacturonic acid (GalA), and galactose and two residues of 3,6-dideoxyxylohexose (Xylhex)."<sup>16</sup> | ||
When ''V. cholerae'' are growing during the logarithmic phase there is little or no change to the cellular envelope. However, towards the end of logarithmic phase and into the begining of stationary phase cholera toxin is produced, which is accompanied by internal swelling of the cell and permeability changes to the cellular envelope.<sup>6</sup> | When ''V. cholerae'' are growing during the logarithmic phase there is little or no change to the cellular envelope. However, towards the end of logarithmic phase and into the begining of stationary phase cholera toxin is produced, which is accompanied by internal swelling of the cell and permeability changes to the cellular envelope.<sup>6</sup> | ||
Anaerobic respiration appears to be the energy producing process for ''V. cholerae'' since the ileum, which is the final portion of the small intestine before entering the colon, is an anaerobic environment. "Alternative electron acceptors such as fumarate, or donors such as formate or glycerol-3-phosphate" are possible players in the metabolism process.<sup>17</sup> | |||
==Ecology== | ==Ecology== | ||
''V. cholerae'' thrives in a water ecology, particularly surface water. The primary connection between humans and pathogenic strains is through water, particularly in economically reduced areas that don't have good water purification systems.<sup>13</sup> | ''V. cholerae'' thrives in a water ecology, particularly surface water. The primary connection between humans and pathogenic strains is through water, particularly in economically reduced areas that don't have good water purification systems.<sup>13</sup> | ||
| Line 89: | Line 90: | ||
As ''V. cholerae'' travels into the lumen it appears that its virulence decreases. Virulence was greatly increased in RpoS mutants compared to its wild-type in virulence-inducing growth conditions. There was a large increase in cholera toxin as well as an increase in fluid output in the ileum | As ''V. cholerae'' travels into the lumen it appears that its virulence decreases. Virulence was greatly increased in RpoS mutants compared to its wild-type in virulence-inducing growth conditions. There was a large increase in cholera toxin as well as an increase in fluid output in the ileum.<sup>12</sup> | ||
| Line 130: | Line 131: | ||
16. Preston, L.M., XU, Q., Johnson, J.A., Joseph, Maneval Jr., D.R., Husain, K., Reddy, G.P., Bush, C.A. AND Morris Jr., J.G. Preliminary Structure Determination of the Capsular Polysaccharide of Vibrio cholerae O139 Bengal Al1837. Journal of Bacteriology. (Feb. 1995); p. 835–838 | 16. Preston, L.M., XU, Q., Johnson, J.A., Joseph, Maneval Jr., D.R., Husain, K., Reddy, G.P., Bush, C.A. AND Morris Jr., J.G. Preliminary Structure Determination of the Capsular Polysaccharide of Vibrio cholerae O139 Bengal Al1837. Journal of Bacteriology. (Feb. 1995); p. 835–838 | ||
17. Xu, Q., Dziejman, M., and Mekalanos, J.J. Determination of the transcriptome of Vibrio cholerae during intraintestinal growth and midexponential phase in vitro. PNAS. (Feb 2003);100(3). 1286-1291. | |||
Edited by Sasha Gardner, student of [mailto:ralarsen@ucsd.edu Rachel Larsen] | Edited by Sasha Gardner, student of [mailto:ralarsen@ucsd.edu Rachel Larsen] | ||
Revision as of 18:40, 5 June 2007
A Microbial Biorealm page on the genus Vibrio cholerae
Classification
Higher order taxa
Bacteria; Proteobacteria; Gammaproteobacteria; Vibrionales; Vibrionaceae; Vibrio
Species
|
NCBI: Taxonomy |
Vibrio cholerae
Description and significance

Vibrio cholerae is a "comma" shaped Gram-negative1 bacteria with a single, polar flagellum for movement. There are numerous strains of V. cholerae, some of which are pathogenic and some of which are not.
The most wide sweeping pathogenic strain is the Vibrio cholerae serotype O1 El Tor N16961 strain that causes the pandemic disease cholera.2 The latest pathogenic serotype O139 was discovered in 1992. The El Tor strain was active in the seventh and most recent pandemic of cholera from 1960's-1970's, as well as in the early 1990's along with serotype O139, both displaying resistance to multiple drugs.7
The bacteria infects the intestine and increases mucous production causing diarrhea and vomiting which result in extreme dehydration and, if not treated, death. It is usually transmitted through the feces of an infected person, often by way of unclean drinking water or contaminated food.2 Since water treatment and sanitation is more advanced in the United States, cholera is not nearly as high of a public health threat in the US as it is in densely populated, economically reduced areas like India or sub-Saharan Africa where water and sewage treatment technology is low.2
It is for this great risk to human health that makes it so worthy of studying and sequencing. And because of the variety of strains, it could be possible to determine the pathogenicity of new strains by comparing their genomes to strains of known pathogenic status.
Filippo Pacini first discovered V. cholerae in Italy in 1854, though it was originally believed to be Robert Koch who discovered it thirty years later in Berlin in 1884.Italians at that time believed that diseases like cholera came from "bad air" or the greek term "miasma." John Snow, a doctor known as the father of epidemiology, did a study during the London cholera epidemic of 1854 from which he concluded that cholera was not passed by bad air but by contaminated water, and discovered that a well that provided water to the public was collecting the leachings of a bacteria laden cesspit. Snow had the handle removed from a water pump that was found to be the neighborhood's source of the contaminated water, and immediately the epidemic began to subside.4, 5
Genome structure
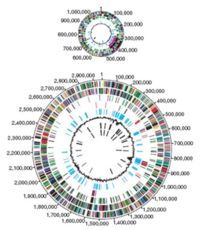
The entire genome of the virulent strain V. cholerae El Tor N16961 has been sequenced,1 and contains two circular chromosomes.3 Chromosome 1 has 2,961,149 base pairs with 2,770 open reading frames (ORF’s) and chromosome 2 has 1,072,315 base pairs, 1,115 ORF’s. It is the larger first chromosome that contains the crucial genes for toxicity, regulation of toxicity and important cellular functions, such as transcription and translation.1
The second chromosome is determined to be different from a plasmid or megaplasmid due to the inclusion of housekeeping and other essential genes in the genome, including essential genes for metabolism, heat-shock proteins and 16S rRNA genes, which are ribosomal sub-unit genes used to track evolutionary relationships between bacteria. Also relevant in determining if the replicon is a chromosome is whether it represents a significant percentage of the genome, and chromosome 2 is 40% by size of the entire genome. And, unlike plasmids, chromosomes are not self-transmissable.3 However it is believed that the second chromosome may have once been a megaplasmid because it contains some genes that are usually found on plasmids.1
V. cholerae contains a genomic island of pathogenicity and is lysogenized with phage DNA. That means that the genes of a virus has become integrated into the bacterial genome and made the bacteria pathogenic.15 The molecular pathway involved in expression of virulence is discussed in the pathology and current research sections below.
Cell structure and metabolism
V. cholerae is Gram-negative has what is called a comma shaped cell body and contains a singular polar flagellum used for motility.1 V. cholerae serogroup O1 does not have a polysaccharide capsule, but serogroup O139 does contain a polysaccaride capsule made up of "N-acetylglucosamine, N-acetylquinovosamine (QuiNAc), galacturonic acid (GalA), and galactose and two residues of 3,6-dideoxyxylohexose (Xylhex)."16
When V. cholerae are growing during the logarithmic phase there is little or no change to the cellular envelope. However, towards the end of logarithmic phase and into the begining of stationary phase cholera toxin is produced, which is accompanied by internal swelling of the cell and permeability changes to the cellular envelope.6
Anaerobic respiration appears to be the energy producing process for V. cholerae since the ileum, which is the final portion of the small intestine before entering the colon, is an anaerobic environment. "Alternative electron acceptors such as fumarate, or donors such as formate or glycerol-3-phosphate" are possible players in the metabolism process.17
Ecology
V. cholerae thrives in a water ecology, particularly surface water. The primary connection between humans and pathogenic strains is through water, particularly in economically reduced areas that don't have good water purification systems.13
Non-pathogenic strains are also present in water ecologies. It is thought that it is the wide variety of strains of pathogenic and non-pathogenic strains that co-exist in aquatic enviornments that allow for so many genetic varieties. Gene transfer is fairly common amongst bacteria and recombination of different V. cholerae genes can lead to new virulent strains.14
Pathology
V. cholerae enters the human body through injestion of contaminated food or water. The bacteria enters the instestine, imbeds itself in the villi of absorptive intestinal cells, and releases cholera toxin. Cholera toxin (CT) is an enterotoxin made up of five B-subunits that form a pore to fits one A-subunit.8 Cholera toxin is made from filamentous phage gene, CTXφ.9 A phage gene is also responsible for another virulence factor of V. cholerae, which is toxin co-regulated pilus (TCP), which acts as a receptor for CTXφ.10
Physiological responses and symptoms that follow release of cholera toxin include stimulation of the mucosal lining of the intestine to secrete fluids. This causes vommiting and watery diarhea that has a "rice water" quality. Death can occur from extreme dehydration and if not treated does occur 50-70% of the time.
Application to Biotechnology
Does this organism produce any useful compounds or enzymes? What are they and how are they used?
The non-toxic B-units of cholera toxin are used for several purposes in cellular and molecular biology.
Current Research
An important area of current research is in quorum sensing, the system by which V. cholerae communicate with each other using molecules called autoinducers. This kind of system enables cells to coordinate a specific function or gene expression as a group. It enables the bacteria to know when others like it are present so that they can team together to do a certain job.
Quorum sensing in V. cholerae has been paralleled to a well known system in V. harveyi, a bacteria that is pathogenic to tiger prawns but not to humans. V. harveyi use quorum sensing to bioluminesce by expression of lux genes. It was shown that luxO represses lux because mutant V. harveyi missing luxO bioluminesced without decreasing in brightness. It was also shown that luxR expresses lux as mutant V. harveyi missing luxR did not bioluminesce at all. Some V. cholerae strains that are not pathogenic are also known to bioluminesce and actually contain lux genes or evolutionary remnants.11
V. cholerae serotype O1 El Tor N16961 is not bioluminescent but is pathogenic and still contains lux genes, including luxO and luxR homologue hapR. This means that virulence may be propagated in V. choleraeby a similar system to the one that propagated bioluminescence in V. harveyi.11 One benefit to this exciting correlation is that virulent pathways in V. cholerae can be visualized by expressing alternate bioluminescent pathways.
In other work, HapR has been found to play a role in what is called the "mucosal escape response." After V. cholerae has found its way to the intestinal cells and secreted toxin, it leaves the cells and enters the watery intestinal lumen. HapR and RpoS are required for this process, and RpoS is an important factor in production of flagellum as well as motility and chemotaxis genes. HapR is also important in motility as the HapR mutant produced regular flagella but had decreased motility from its wild-type, or its unmutated form.12
As V. cholerae travels into the lumen it appears that its virulence decreases. Virulence was greatly increased in RpoS mutants compared to its wild-type in virulence-inducing growth conditions. There was a large increase in cholera toxin as well as an increase in fluid output in the ileum.12
These findings suggest that HapR and RpoS help V. cholerae detach from intestinal cells and move through the intestinal lumen, and that RpoS reduces the amount of cholera toxin that is produced as the bacteria is traveling through the lumen.12 Perhaps this is to conserve energy as it is traveling to its next destination where it will increase in virulence upon implantation in another gastrointestinal system.
Most recently another protein, Fis, has been discovered to play an additional role in quorum sensing in V. cholerae. Fis regulates HapR in the same pathway as luxO, though possibly further upstream. It was determined that "fis expression is induced at an early exponential-phase and declines in stationary phase."13 This means that fis must play a direct role in the production of cholera toxin and therefore regulation of virulence.6
References
1. Heidelberg, J.F., Eisen, J.A., Nelson, W.C., et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. (2000);406 (6795), 477-483.
2. Center for Disease Control, Coordinating Center for Infectious Diseases / Division of Bacterial and Mycotic Diseases, October 6, 2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/cholera_g.htm
3. Trucksis, M., Michalski, J., Deng, Y.K., Kaper, J.B. The Vibrio cholerae genome contains two unique circular chromosomes. Proc. Natl Acad. Sci. USA (1998);95, 14464-14469.
4. Bentivoglio, M., Pacini, P. Filippo Pacini: a determined observer. Brain Res Bull. (1995);38(2):161-5.
5. Howard-Jones N. Robert Koch and the cholera vibrio: a centenary. British Medical Journal. (1984);288, 379-381.
6. Kennedy, J.R., Stephen, H.R. Fine Structure of Vibrio cholerae During Toxin Production. J Bacteriol. (December 1969);100(3): 1393–1401.
7. Ingole, K.V., Jalgaonkar, S.V., Fule, C., Fule, R.P. Changing pattern of Vibrio cholerae serotype EL TOR and 0139 in Yavatmal (Maharashtra, India) during 1992 to 1994. Indian J Pathol Microbiol. (July 1997);40(3):369-71.
8. Zhang R., Scott D., Westbrook M., Nance S., Spangler B., Shipley G., Westbrook E. (1995). The three-dimensional crystal structure of cholera toxin. J Mol Biol 251 (4): 563-73.
9. Davis B., Waldor M. Filamentous phages linked to virulence of Vibrio cholerae. Curr Opin Microbiol. (2003);6 (1): 35-42.
10. Boyd, E.F., Waldor, M.K. Evolutionary and functional analyses of variants of the toxin-coregulated pilus protein TcpA from toxigenic Vibrio cholerae non-O1/non-O139 serogroup isolates. Microbiology. (2002);148, 1655-1666.
11. Miller, M.B., Skorupski, K., Lenz, D.H., Taylor, R.K., Bassler, B.L. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. (2002);110, 303-314.
12. Nielsen, A.T., Dolganov, N.A., Otto, G., Miller, M.C., Wu, C.Y., Schoolnik, G.K. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog. 2006 Oct;2(10):e109
13. Faruque, S.M., Albert, M.J., Mekalanos, J.J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. (1998 Dec);62(4):1301-14
14. Faruque, S.M., Nair, G.B. Molecular ecology of toxigenic Vibrio cholerae. Microbiol Immunol. (2002);46(2):59-66.
15. Schaechter, M., Ingraham, J.L., Neidhardt, F.C. Microbe. ASM Press, Washington D.C. 2006.
16. Preston, L.M., XU, Q., Johnson, J.A., Joseph, Maneval Jr., D.R., Husain, K., Reddy, G.P., Bush, C.A. AND Morris Jr., J.G. Preliminary Structure Determination of the Capsular Polysaccharide of Vibrio cholerae O139 Bengal Al1837. Journal of Bacteriology. (Feb. 1995); p. 835–838
17. Xu, Q., Dziejman, M., and Mekalanos, J.J. Determination of the transcriptome of Vibrio cholerae during intraintestinal growth and midexponential phase in vitro. PNAS. (Feb 2003);100(3). 1286-1291.
Edited by Sasha Gardner, student of Rachel Larsen