Host Dependency of Mycobacterium leprae: Difference between revisions
No edit summary |
|||
| Line 32: | Line 32: | ||
<i>M. leprae</i> has the genes for the proteins for the Krebs' cycle and glyoxylate cycle, but the organism essentially relies on a single electron donor, FADH. The 2nd figure to the left shows respiration pathways for <i>M. leprae</i> compared to <i>M. tuberculosis</i>. As in the previous figure, pathways in black are present in both <i>M. leprae</i> and <i>M. tuberculosis</i>, grey pathways are pathways that exist in <i>M. tuberculosis</i> that <i>M. leprae</i> still has pseudogenes for. | <i>M. leprae</i> has the genes for the proteins for the Krebs' cycle and glyoxylate cycle, but the organism essentially relies on a single electron donor, FADH. The 2nd figure to the left shows respiration pathways for <i>M. leprae</i> compared to <i>M. tuberculosis</i>. As in the previous figure, pathways in black are present in both <i>M. leprae</i> and <i>M. tuberculosis</i>, grey pathways are pathways that exist in <i>M. tuberculosis</i> that <i>M. leprae</i> still has pseudogenes for. NADH, one of the more common electron donors involved in metabolism in many species, is not used for electron transport in <i>M. leprae</i>. This causes aerobic respiration to be much less efficient. There are no anaerobic respiration pathways in <i>M. leprae</i>, so the bacteria have to make due with a less efficient aerobic pathway. This makes the bacteria even harder to culture due to the difficulty of composing a media with the correct levels of oxygen and the other necessary vitamins and minerals to permit growth. <i>M. leprae</i> has evolved to only survive in it's niche environment with a host. | ||
| Line 42: | Line 42: | ||
<i>Mycobacterium leprae</i> primarily infects the lower temperature extremities, such as the epithelial cells and nonmyelin producing Schwann cells around peripheral nerves in the hands and feet, and occasionally the upper respiratory tract, testes, and cornea, causing the disease leprosy. The symptoms of leprosy include anesthetic skin lesions and enlarged peripheral nerves. Infected areas witness less sensation to pain and temperature. Infection usually follows breathing in through the nose microscopic droplets excreted from an infected individual containing <i>M. leprae</i>, usually from sneezing. There are also cases of infection when a human comes into contact with soil containing <i>M. leprae</i>. Skin contact with an infected individual has not proven to pass on the infection [OOI]. The number of cases of individuals infected with leprosy have decreased overtime with the help of antibiotics, but there are still hundreds of thousands of cases of leprosy across the world. | <i>Mycobacterium leprae</i> primarily infects the lower temperature extremities, such as the epithelial cells and nonmyelin producing Schwann cells around peripheral nerves in the hands and feet, and occasionally the upper respiratory tract, testes, and cornea, causing the disease leprosy. The symptoms of leprosy include anesthetic skin lesions and enlarged peripheral nerves. Infected areas witness less sensation to pain and temperature. Infection usually follows breathing in through the nose microscopic droplets excreted from an infected individual containing <i>M. leprae</i>, usually from sneezing. There are also cases of infection when a human comes into contact with soil containing <i>M. leprae</i>. Skin contact with an infected individual has not proven to pass on the infection [OOI]. The number of cases of individuals infected with leprosy have decreased overtime with the help of antibiotics, but there are still hundreds of thousands of cases of leprosy across the world. | ||
It has been shown that <i>M. leprae</i> are able to bind to nasal epithelial cells by binding to a soluble protein, fibronectin, that binds to fibronectin receptors on the surface of the epithelial cell [Byrd]. It is thought that <i>M. leprae</i> then enter the nasal epithelial cells, enter the blood stream, and migrate to places with the best environment, the nonmyelinating Schwann cells in the extremities. It was shown that <i>M. leprae</i> invade the nonmyelinating Schwann cells and multiply, as well as attach to myelinating Schwann cells. The <i>M. leprae</i> cells release PGL proteins, which are thought to disrupt the DRP2-dystroglycan complex in the Schwann cell, causing the myelination to deteriorate. Myelinating Schwann cells produce DRP2, which is necessary for myelination. The dystroglycan complexes link the | It has been shown that <i>M. leprae</i> are able to bind to nasal epithelial cells by binding to a soluble protein, fibronectin, that binds to fibronectin receptors on the surface of the epithelial cell [Byrd]. It is thought that <i>M. leprae</i> then enter the nasal epithelial cells, enter the blood stream, and migrate to places with the best environment, the nonmyelinating Schwann cells in the extremities. It was shown that <i>M. leprae</i> invade the nonmyelinating Schwann cells and multiply, as well as attach to myelinating Schwann cells. The <i>M. leprae</i> cells release PGL proteins, which are thought to disrupt the DRP2-dystroglycan complex in the Schwann cell, causing the myelination to deteriorate. Myelinating Schwann cells produce DRP2, which is necessary for myelination. The dystroglycan complexes link the Schwann cells and are thought to carry signals from the inside of the cell to the outside [ooi]. It has been shown that dead <i>M. leprae</i> cells or the cell wall alone can still cause demyelination to occur [Brophy]. This means that, even after antibiotics are administered and the bacteria are killed, more demyelination occurs. This is why it is important that the infection is treated right away; before too much damage to the nervous system is done. | ||
<i>M. leprae</i> have been also noted to cause inflammation in the extremities of infected individuals by turning off the immune system. On the cell wall of <i>M. leprae</i> there are lipids that suppress T-cell activation without altering the membrane of the T cells [Moura]. By inhibiting the immune system, <i>M. leprae</i> is able continue to thrive without being attacked. It is because of <i>M. leprae</i>'s ability to disarm the immune system that the infection is extremely hard to get rid of naturally. Before the time of antibiotics, the majority of the individuals that became infected and showed symptoms of the disease usually died from it. | <i>M. leprae</i> have been also noted to cause inflammation in the extremities of infected individuals by turning off the immune system. On the cell wall of <i>M. leprae</i> there are lipids that suppress T-cell activation without altering the membrane of the T cells [Moura]. By inhibiting the immune system, <i>M. leprae</i> is able continue to thrive without being attacked. It is because of <i>M. leprae</i>'s ability to disarm the immune system that the infection is extremely hard to get rid of naturally. Before the time of antibiotics, the majority of the individuals that became infected and showed symptoms of the disease usually died from it. | ||
| Line 49: | Line 49: | ||
<br> | <br> | ||
Depending on the host, <i>Mycobacterium leprae</i> | Depending on the host, the severity of an infection of <i>Mycobacterium leprae</i> can range from no infection to an extreme case of leprosy. How well the bacteria grow in the body depends on the genetics of the host. Some individuals are more susceptible than others, for genetic reasons ranging from COME BACK | ||
discuss different types of infections | discuss different types of infections | ||
| Line 70: | Line 70: | ||
[] Ascenzi, P., Bolognesi, M., Visca, P. NO Dissociation Represents the Rate Limiting step for O2-Mediated Oxidation of Ferrous Nitrosylated Mycobacterium leprae truncated hemoglobin O. BBRC, 357. 2007. 809-814. | [] Ascenzi, P., Bolognesi, M., Visca, P. NO Dissociation Represents the Rate Limiting step for O2-Mediated Oxidation of Ferrous Nitrosylated Mycobacterium leprae truncated hemoglobin O. BBRC, 357. 2007. 809-814. | ||
[ Brophy, P. Subversion of Schwann cells and the leper's bell. Science 296. 2002. 862-863 | |||
[] http://images.google.com/imgres?imgurl=http://www.stanford.edu/group/parasites/ParaSites2008/Eric%2520Chow_Tania%2520Roman/Leprosy_files/image002.jpg&imgrefurl=http://www.stanford.edu/group/parasites/ParaSites2008/Eric%2520Chow_Tania%2520Roman/Leprosy.htm&usg=__FuMa0EbNt90SLHIsPNtHoPG_L-U=&h=282&w=415&sz=37&hl=en&start=12&sig2=QhgkDewUDwUHwVGbYZ7zJQ&tbnid=SluTAPufmlK5lM:&tbnh=85&tbnw=125&prev=/images%3Fq%3Dmycobacterium%2Bleprae%26gbv%3D2%26hl%3Den%26sa%3DG&ei=sR7mSd6pK5T0nQfQ4v2XDg'' | [] http://images.google.com/imgres?imgurl=http://www.stanford.edu/group/parasites/ParaSites2008/Eric%2520Chow_Tania%2520Roman/Leprosy_files/image002.jpg&imgrefurl=http://www.stanford.edu/group/parasites/ParaSites2008/Eric%2520Chow_Tania%2520Roman/Leprosy.htm&usg=__FuMa0EbNt90SLHIsPNtHoPG_L-U=&h=282&w=415&sz=37&hl=en&start=12&sig2=QhgkDewUDwUHwVGbYZ7zJQ&tbnid=SluTAPufmlK5lM:&tbnh=85&tbnw=125&prev=/images%3Fq%3Dmycobacterium%2Bleprae%26gbv%3D2%26hl%3Den%26sa%3DG&ei=sR7mSd6pK5T0nQfQ4v2XDg'' | ||
Revision as of 02:46, 16 April 2009
Introduction
Mycobacterium leprae, the bacterial cause of leprosy, is almost impossible to culture in a laboratory. M. leprae is an acid fast gram positive bacillus, and can only grow when acting as a parasite in animals with lower body temperature, such as armadillos, genetically immune deficient mice, or the extremities of a human body (1). M. leprae has one of the slowest doubling times of any pathogen; it takes approximately 14 days for the cells to divide (1). M. leprae is easily detected on Fite-Faraco staining. M. leprae is from the same genus as Mycobacterium tuberculosis; the two species have similar physical characteristics and similar genomes.
The genome for M. leprae has been sequenced, and it has been found that almost half of the genes are pseudogenes; genes that no longer code for proteins to be transcribed in the cell. Many of these pseudogenes correspond to genes found in Mycobacterium tuberculosis that are still functional (1). The loss of these genes have caused M. leprae to rely on the host cell to survive; the bacteria needs an extremely specific environment to thrive in.
M. leprae has plagued mankind since ancient times. Antibiotics have helped to decrease the number of cases of leprosy, but poorer areas around the world still have problems with this disease. In 2004, there were approximately 50,000 new cases of infection.
Mycobacterium leprae genome; missing genes in M. leprae compared to M. tuberculosis
1.Talk about the genome.
2. Discuss differences between M tuberculosis and M leprae use the two figures
define pseduogene, inablility to grow in media even with all of the added things
The Mycobacterium leprae genome was sequenced by S. T. Cole and was published in Nature in 2001 []. His group found that the genome consisted of a single circular chromosome approximately 3,268,203 bp. The G-C content of the genome is 57.7%, and only 49.5% of the genome still codes for proteins []. The genomes for M. leprae and M. tuberculosis are quite similar, and about 27% of M. leprae's genome are pseudogenes that directly correspond to genes still expressed in M. tuberculosis []. The remaining 23% of the genome does not resemble coding genes; these sections seem to have mutated over time and are currently unrecognizable. The similarity of the pseudogenes in M leprae compared to expressed genes in M. tuberculosis suggest that the genome of M laprae has evolved to lose these genes because they were not necessary for survival. This is an example of reductive evolution. It is estimated that M laprae has lost approximately 2000 genes from its genome []. The loss of genes in metabolic pathways such as energy metabolism, limiting the carbon sources M laprae can use, and holes in respiration pathways help to explain why M laprae can not be cultured in a laboratory and has only been shown to infect humans, the footpads of mice, and armadillos.
From a thorough examination of the genome of M laprae, it was found that M laprae does have all the main metabolic and biosynthetic pathways, but a there are some missing genes. Compared to the genome of M tuberculosis, M laprae lacks the genes for vitamin B12 synthesis and metC, a gene that codes for the enzyme that converts cysteine into methionine. This means that M laprae can not synthesize the vitamin or methionine, an amino acid, and instead has to be taken from the environment. to test the presence of Methionine and vitamin B12 were enough for M laprae to grow, an attempt to culture the bacteria in media containing these molecules was not successful.
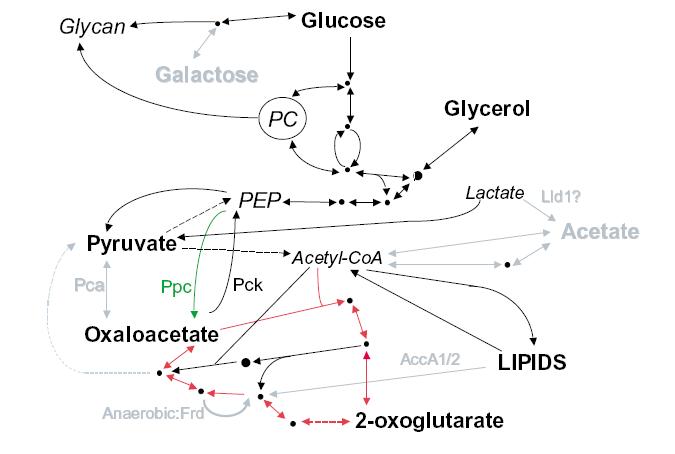
There are significant losses in the genome of M. leprae for carbon and energy metabolism. All the central pathways are present; M. leprae metabolizes glucose, but other carbon sources can not be metabolized for energy. There are pseudogenes present in M. leprae corresponding to genes in M. tuberculosis for proteins that enable the bacteria to breakdown other carbon sources such as acetate and galactose. The diagram on the right shows carbon metabolism in M. leprae compared to M. tuberculosis. Pathways in black are present in both M. leprae and M. tuberculosis, grey pathways are pathways that exist in M. tuberculosis that M. leprae still has pseudogenes for. Bolded carbon sources are possible for use by M. leprae, grey carbon sources are unusable by M. leprae. The pathway with the red arrows is the Krebs cycle. Not being able to use these sources for energy means that M. leprae has to rely much more heavily on using glucose, which is not found in the environment as commonly as other carbon sources. Most microbes are able to convert other sources such as galactose into glucose, but M. leprae is not able to do this and must rely on another organism to make sure it has enough glucose.
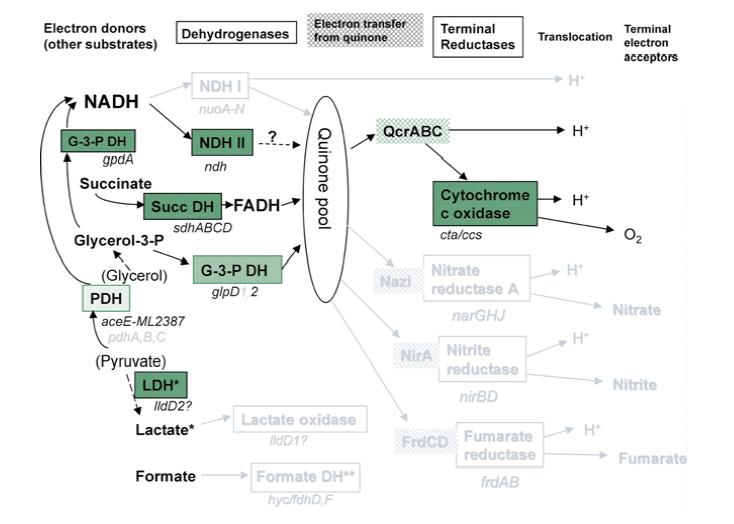
M. leprae has the genes for the proteins for the Krebs' cycle and glyoxylate cycle, but the organism essentially relies on a single electron donor, FADH. The 2nd figure to the left shows respiration pathways for M. leprae compared to M. tuberculosis. As in the previous figure, pathways in black are present in both M. leprae and M. tuberculosis, grey pathways are pathways that exist in M. tuberculosis that M. leprae still has pseudogenes for. NADH, one of the more common electron donors involved in metabolism in many species, is not used for electron transport in M. leprae. This causes aerobic respiration to be much less efficient. There are no anaerobic respiration pathways in M. leprae, so the bacteria have to make due with a less efficient aerobic pathway. This makes the bacteria even harder to culture due to the difficulty of composing a media with the correct levels of oxygen and the other necessary vitamins and minerals to permit growth. M. leprae has evolved to only survive in it's niche environment with a host.
Interaction with host cells
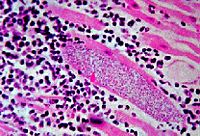
Mycobacterium leprae primarily infects the lower temperature extremities, such as the epithelial cells and nonmyelin producing Schwann cells around peripheral nerves in the hands and feet, and occasionally the upper respiratory tract, testes, and cornea, causing the disease leprosy. The symptoms of leprosy include anesthetic skin lesions and enlarged peripheral nerves. Infected areas witness less sensation to pain and temperature. Infection usually follows breathing in through the nose microscopic droplets excreted from an infected individual containing M. leprae, usually from sneezing. There are also cases of infection when a human comes into contact with soil containing M. leprae. Skin contact with an infected individual has not proven to pass on the infection [OOI]. The number of cases of individuals infected with leprosy have decreased overtime with the help of antibiotics, but there are still hundreds of thousands of cases of leprosy across the world.
It has been shown that M. leprae are able to bind to nasal epithelial cells by binding to a soluble protein, fibronectin, that binds to fibronectin receptors on the surface of the epithelial cell [Byrd]. It is thought that M. leprae then enter the nasal epithelial cells, enter the blood stream, and migrate to places with the best environment, the nonmyelinating Schwann cells in the extremities. It was shown that M. leprae invade the nonmyelinating Schwann cells and multiply, as well as attach to myelinating Schwann cells. The M. leprae cells release PGL proteins, which are thought to disrupt the DRP2-dystroglycan complex in the Schwann cell, causing the myelination to deteriorate. Myelinating Schwann cells produce DRP2, which is necessary for myelination. The dystroglycan complexes link the Schwann cells and are thought to carry signals from the inside of the cell to the outside [ooi]. It has been shown that dead M. leprae cells or the cell wall alone can still cause demyelination to occur [Brophy]. This means that, even after antibiotics are administered and the bacteria are killed, more demyelination occurs. This is why it is important that the infection is treated right away; before too much damage to the nervous system is done.
M. leprae have been also noted to cause inflammation in the extremities of infected individuals by turning off the immune system. On the cell wall of M. leprae there are lipids that suppress T-cell activation without altering the membrane of the T cells [Moura]. By inhibiting the immune system, M. leprae is able continue to thrive without being attacked. It is because of M. leprae's ability to disarm the immune system that the infection is extremely hard to get rid of naturally. Before the time of antibiotics, the majority of the individuals that became infected and showed symptoms of the disease usually died from it.
Genetic component of the host
Depending on the host, the severity of an infection of Mycobacterium leprae can range from no infection to an extreme case of leprosy. How well the bacteria grow in the body depends on the genetics of the host. Some individuals are more susceptible than others, for genetic reasons ranging from COME BACK
discuss different types of infections
some people more susceptible then others, use the three papers. Talk about PARK2, PACRG
Conclusion
Discuss how outbrakes are still a problem worldwide, what drugs we use to combat them, link to the lepra website.
References
[1] Slonczewski, J., Foster, J. Microbiology: An Evolving Science. New York: W. W. Norton, 2009. 700-702.
[] Ooi, W., Srinivasan, J. Leprosy and the Peripheral Nervous System: Basic and Clinical Aspects. Muscle and Nerve, October 2004. 393-409.
[] Schurr, E., Alcais, A., Singh M., Mehra, N., Abel, L. Mycobacterial infections: PARK2 and PACRG associations in Leprosy. Tissue Antigens, 2007. 231-233.
[] Geluk, A., Ottenhoff, T. HLA and Leprosy in the Pre and Postgenomic Eras. Human Immunology, 67. 2006. 439-445.
[] Wheeler, P. Leprosy- Clues about the Biochemistry of Mycobacterium leprae and its Host-Dependency from the Genome. World Journal of Microbiology and Biochemistry, 19. 2003. 1-16.
[] Ascenzi, P., Bolognesi, M., Visca, P. NO Dissociation Represents the Rate Limiting step for O2-Mediated Oxidation of Ferrous Nitrosylated Mycobacterium leprae truncated hemoglobin O. BBRC, 357. 2007. 809-814.
[ Brophy, P. Subversion of Schwann cells and the leper's bell. Science 296. 2002. 862-863
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.