Malaria (Plasmodium falciparum) in sub-Saharan Africa: Difference between revisions
| Line 70: | Line 70: | ||
[9] [http://www.who.int/features/factfiles/malaria/en/index.html "WHO | 10 facts on malaria." WHO | World Health Organization. Web. 18 Aug. 2009.] | [9] [http://www.who.int/features/factfiles/malaria/en/index.html "WHO | 10 facts on malaria." WHO | World Health Organization. Web. 18 Aug. 2009.] | ||
[10] Gardner, Malcolm J., et al. “Genome sequence of the human malaria parasite Plasmodium Falciparam.” Nature. 2002. Volume 419. p. 498-511. http://www.ncbi.nlm.nih.gov/pubm/12368864?ordinalpos=itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.SmartSearch&log$=citationsensor | |||
Edited by Andy Chen, Alice Nguyen, Kris Vasant, Chirag Yadav, Matthew Hsia, Renu Gaur, and Amreeta Panesar, students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | Edited by Andy Chen, Alice Nguyen, Kris Vasant, Chirag Yadav, Matthew Hsia, Renu Gaur, and Amreeta Panesar, students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | ||
Revision as of 23:38, 27 August 2009
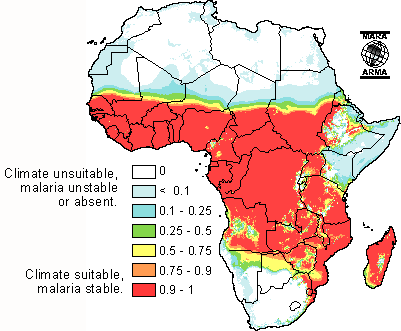
Introduction
Malaria is an infectious disease caused by the parasitic protozoan Plasmodium, which can only be transferred by the female Anopheles mosquito. Malaria is spread when the mosquito bites into a person who is already infected. The parasites from the blood uptake reproduce in the mosquito and mix with the saliva so that the next time the mosquito bites another person, parasites are transferred.[1]
There are four parasitic protozoans which cause malaria: Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax. Of these parasites, Plasmodium falciparum is the most dangerous and can cause coma or death.[2] Symptoms include high fever, chills, vomiting, and nausea and they don’t appear until 10-15 days after the initial mosquito bite.[3]
Malaria, which is Italian for “bad air,” has been infecting humans since the last Ice Age, more than 20,000 years ago in sub-Saharan Africa and the Mediterranean region.[4] It was first documented in Chinese medical scripts in 2700 BCE and later in Greece in the 4th century BCE. It was then that Roman writers attributed malaria to swampy areas. The discovery of a tree bark that remedies malaria was discovered in the early 17th century – Spanish missionaries in South America were introduced to the bark by local Indian tribes to cure fevers. The medicine in the bark, now called quinine, is currently being used as anti-malarial drug.[5]
The World Health Organization estimates that 300-500 million cases of malaria occur annually worldwide and more than one million victims are killed every year. About 90% of these deaths are from sub-Saharan Africa, occurring mostly in children under the age of 5.[6] 50,000 deaths occur in Zambia alone. 1 in every 5 children in Africa die from malaria, and costs Africa’s gross domestic product approximately $12 billion per year in lost wages due to debilitating effects of the disease.[7][8] Although malaria is preventable and curable, misuse of antimalarial drugs over the past century led to parasitic resistance to these malaria medications.[9]
Genome of Plasmodium falciparum
There are four species of genus Plasmodium that infect humans; Plasmodium falciparum being the most lethal and virulent. P. falciparum is characterized as a protozoan, a single celled eukaryotic organism. Comparative genome analysis amongst eukaryotes revealed that it is more similar to Arabidopsis thaliana than to any other taxa. Furthermore, 237 P. falciparum proteins strongly matched to proteins in all completed eukaryotic genomes but no matches against prokaryotic proteome, suggesting eukaryotic cell lineage. The proteins that were used for comparison included ones that had contributed to cytoskeleton construction and maintenance, cell cycle regulation, translation, replication and many other functions. These proteins were thus used to differentiate between eukaryotes and prokaryotes.
Using a whole chromosome shotgun sequencing strategy, the nuclear genome of P. falciparum 3D7 was sequenced. It is composed of 22.8 megabases (Mb), distributed among 14 chromosomes ranging from 0.643 to 3.29Mb. 80.6% of the genome is adenosine and thymine rich while 20.4% is guanine and cytosine rich. This percentage increases to about 90% in introns and intergenic regions. It is predicted that 54% of P. falciparum genome are introns. Excluding introns, the mean length of the genome is 2.3kb. Many of the large genes present in the genome do not possess recognizable signal peptides thus cannot be identified. With the presence of large genes, more research needs to be done to identify these genes that encode for proteins that do not possess recognizable signal peptides. Also, centromeres have not been identified.
On the contrary to most other eukaryotic organisms, the P. falciparum genome does not carry long tandem repeated arrays of ribosomal RNA (rRNA) genes. Instead, numerous single 18s-5.8s-28s rRNA units are present on the 14 chromosomes. Unlike many other eukaryotes, the P. falciparum genome does not contain long tandem repeated arrays of ribosomal RNA (rRNA) genes. Instead, Plasmodium parasites contain several single 18S-5.8S-28S rRNA units distributed on different chromosomes. The relevance of this is that the sequence encoded by one rRNA in one unit differs from another unit, thus revealing regulatory development, which can lead to different expression of rRNA that alter cell growth and patterns of cell development. For example, the S-type rRNA gene is expressed in the mosquito vector while the A-type rRNA is expressed in the human host.
Chromosome length is further varied by the subtelomeric regions. Subtelomeric regions are proving to be important in research due to the fact that they exhibit antigenic variation. Telomeres are important in P. falciparum because they inhabit genes necessary for immune evasion. One of those important gene families is termed as var which encodes for the erythrocyte membrane protein which are exported to the surface of the infected red blood cells. This mediates adhesion to host endothelial receptors, causing the separation of infected cells from different organs. This is important to the virulence and to the development of severe disease. The other two gene families are rif (repetitive interspersed family) and stevor (sub-telomeric variable open reading frame).
Of the 5,268 predicted proteins, 733 were identified as enzymes important for chemical reactions and 208 genes are known to be involved in the evasion of the host immune system. On the other hand, about 60% of the proteins do not show similarity to proteins of other organisms thus underlying these proteins to be purely unique. This is a high proportion not observed in other eukaryotes. Still, these differences do not necessarily imply that fewer malaria genes are involved in these processes, but highlight areas of malaria biology where knowledge is limited. [10]
Transmission of Plasmodium falciparum into the host cell
Drug Treatment
Why is this disease a problem in sub-Saharan Africa
Do lifestyle/environment/economics/political issues play a role?
What is being done to address this problem
Include anything being done by the local government or groups as well as efforts by non-local groups.
What else could be done to address this problem
Are there solutions that could be successful but haven't been implemented due to political or economic reasons? Are there successful efforts in other countries? Are there reasons why these efforts may or may not be successful in the country you've focused on? etc. etc.
Because anti-malaria drugs and vaccines are so expensive and limited, it’s important to invest money into diagnostic products that are effective and accurate. Misdiagnosis of malaria leads to too many prescriptions of limited drugs and vaccines. In the past couple of months, 30.8% of the patients were misdiagnose and 29.3% were given drugs they didn’t need [7]. With microscopy and the rapid diagnostic test (RDT), patients can be diagnose accurately. Diagnosis of malaria is 93.5% accurate with microscopy and 97.5% accurate with RDT [8]. RDT detects the parasitic antigens that are present in malaria quicker and more accurate than microscopy[9]. Although RDT is more expensive than microscopy, it needs to be implemented in areas that have severe cases of malaria. Since the diagnosis are quick and accurate, treating patients with malaria will be more cost efficient and there will be more vaccines and anti-malaria drugs to be used efficiently. Overtime the money saved on misdiagnosed patients will cover the costs of RDT [10]. In the absence of microscopy and RDT, pregnant women and children five and younger with high fevers are automatically given anti-malaria drugs because they are more prone to malaria.
References
[1] "WHO | Q&A: malaria." WHO | World Health Organization. Web. 18 Aug. 2009.
[2] "WHO | Malaria." WHO | World Health Organization. Jan. 2009. Web. 18 Aug. 2009.
[3] "WHO | Malaria." WHO | World Health Organization. Web. 18 Aug. 2009.
[7] "Africa - Striking Back at Malaria in Sub-Saharan Africa." The World Bank. Web. 18 Aug. 2009.
[9] "WHO | 10 facts on malaria." WHO | World Health Organization. Web. 18 Aug. 2009.
[10] Gardner, Malcolm J., et al. “Genome sequence of the human malaria parasite Plasmodium Falciparam.” Nature. 2002. Volume 419. p. 498-511. http://www.ncbi.nlm.nih.gov/pubm/12368864?ordinalpos=itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.SmartSearch&log$=citationsensor
Edited by Andy Chen, Alice Nguyen, Kris Vasant, Chirag Yadav, Matthew Hsia, Renu Gaur, and Amreeta Panesar, students of Rachel Larsen
This template is just a general guideline of how to design your site. You are not restricted to this format, so feel free to make changes to the headings and subheadings and to add or remove sections as appropriate.
