Haemophilus influenzae in Developing Countries: Difference between revisions
No edit summary |
|
(No difference)
| |
Revision as of 20:12, 2 April 2010
Introduction
Haemophilus influenzae was first described by Richard Pfeiffer in 1982 during the outbreak of influenza. The name of this bacterium was given by Winslow, et al. in 1920. [3] Haemophilus influenzae was the leading cause of death worldwide before the introduction of an effective vaccine in 1990’s. It is the main cause of bacterial meningitis in many developing countries. H.influenzae. characterizes a group of bacteria that may cause different types of clinical syndromes in infants and children under the age of five. H. influenzae can cause ear, eye, or sinus infections, pneumonia, meningitis, and a life threatening infection called epiglottises. In addition to the deadly disease, one of the most significant factors that prevents many developing countries from controlling or curbs the Hib vaccine is the lack of awareness of the Hib conjugate vaccine.[1,2]
Furthermore, in 1995, Haemophilus influenzae was the first free-living organism to have its entire chromosome sequenced, just ahead of E.coli, because the H.influenzaegenome is smaller in size than E.coli. [4]
Pathogenesis of Haemophilus influenzae
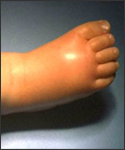
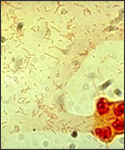
Haemophilus influenzae is an invasive disease caused by a bacterium called Haemophilus influenzae which infects infants and children under the age of five. In addition, Haemophilus influenzae diseases include clinical syndrome of meningitis (an infection of the membranes that surround the brain and spinal cord), bacteremia or sepsis, epiglottitis (an infection of the area of the throat that covers and protects the voice and trachea during swallowing), pneumonia, septic arthiritis, osteomyelitis, pericarditis, and cellulitis. Moreover, syndromes of mucosal infections such as bronchitis, sinusitis, and otitis media (middle ear infection) are considered noninvasive diseases. [5,2] Haemophilus influenzae is a small (1µmx0.3µm), pleomorphic, gram-negative coccobacillus that is a non-motile, non-spore-forming, fastidious, facultative anaerobe; although it generally grows as an aerobe. [6]
Structure and Optimum Living Environment
Microbewiki Link: Haemophilus influenzae
The outermost structure of H.influenzae is composed of polyribosyl-ribitol phosphate (PRP), a polysaccharide that is the most responsible for virulence (type b) and immunity. H. Influenzae requires two erythrocyte factors for growth: X factor (hemin) and V factor (nicotinamide-adenine-dinucleotide, NAD or NADP). These factors are released after the red blood cell lyses. [6] Moreover, the bacterium grows best at 35-37ºC and has an optimum pH of 7.6. In the laboratory, Hi is usually grown on chocolate blood agar which is prepared by adding to an agar base at 80ºC where the heat releases X and V factors from the RBC’s and turns the medium a chocolate brown color. [4]
Transmission of H.influenzae
The organism enters the body through the nasopharynx. Organisms colonize the nasopharynx and may remain in the body for several months with no symptoms (asymptomatic carrier). H. influenzae bacteria usually live in the upper respiratory tract and is transmitted by direct contact with an infected person or by inhalation of respiratory tract droplets when coughing or sneezing. [3,7] The symptoms related to Hib infection include fever, lethargy, vomiting, and stiff neck. [20] Haemophilus influenzae may be either encapsulated (typeable) or un-encapsulated (non-typeable). It is divided into six different antigenic capsular serotypes (a-f) which can cause persistent diseases in an infected person. Non-typeable strains can also cause invasive disease but are less virulent than the encapsulated strains and only cause infrequent infections in children. [5] Moreover, H. influenzae is vulnerable to lysis by antibodies. Anticapsular antibodies promote phagocytosis and bacteriolysis. During meningitis, phagocytosis is the main host defense mechanism against H.influenzae. The inhabitant bacteria invade the mucosa and enter the bloodstream. The antiphagocytic nature of the Hib capsule and the absence of the anticapsular antibody leads to higher levels of bacterial proliferation. Whenever the bacterial concentration exceeds a critical level, it can disseminate to various sites, including meninges, joints, and lungs.[4] Additionally, newborns have low risk of infection because of the acquired maternal antibodies. When these transplacental antibodies to the polyribosyl-ribitol phosphate (PRP) antigen diminish, infants are at high risk of developing invasive H.influenzaedisease, and their immune responses are low even after the disease. Therefore, infants are at high risk of repeat infections since prior episodes of H.influenzaedo not present immunity. [6]
Treatments and Solutions
Components of the Conjugate Vaccine
"The Hib vaccine is classified as a polysaccharide conjugate vaccine, which is a type of inactivated bacterial vaccine. It is made by joining a piece of the polysaccharide capsule that surrounds the Hib bacterium to a protein carrier. This joining process is called conjugation. Conjugating a protein carrier to a piece of the polysaccharide capsule from a Hib bacterium creates an effective vaccine".[22]
Four types of Vaccines
There are four main types of conjugate vaccines that are used with different carrier proteins and have been permitted to used in infants.[17]
1.PRP-D(ProHIBit)- it has a Hib-purified polyribosyribitol phosphate in combination with the diphtheria toxoid. Only infants greater than a year old may use this type.[18]
2.PRP-OMP(PedvaxHIB) – has Hib-PRP that is combined with the protein that’s on the outer membrane of Neisseria meningitis group b. This is a series of shots that are given at ages
of 2, 4, 12 months.[18]
3.HbOC(HibTITTER)- contains a part of the Hib polysaccharide combined with the mutant Crm-197 diphtheria protein.[18]
4.PRP-T(OmniHib/ActHIB) has PRP combined with tetanus toxoid. a.Are given in a series from ages of 2, 4, 6, 12 to 15 months of age.[18]
Effectiveness of the Vaccines
One study, children less than 5 years old were evaluated for the effectiveness of the conjugate vaccines in preventing Hib, variation in efficacy depending on the type of vaccine, number of doses, age when received first dose, and any unfavorable side effects of the vaccine.[19] Of all the factors tested, none of them seemed to be significant and no critical negative effects were observed. The study concluded that the HIB vaccine is risk-free as well as effectual in preventing disease. The author also states that if the vaccine is to be used in developing countries they have to consider two main factors, which is its daunting expenses and how to deliver the vaccine cost-effectively.[17]
Moreover, a very similar study was performed in the country of Uganda. The study was to determine the overall benefit of introducing the vaccine into this country and measure how effective the conjugate vaccine is. Some children who were affected with acute bacterial meningitis and children just under 5 years of age were surveyed for 6 years. Before the vaccine, there were 88 incidences per 100,000 children and this staggering number dropped to a low number of 13 affected children- all within 4 years. The results were indeed convincing and the Hib disease was essentially wiped out of Uganda within 5 years. Therefore, in order to sustain the viable benefits of the vaccine for a long period of time, there must be enough funding, excellent surveillance, and a health department to effectively deliver the vaccine.[19]
Why is H.influenzae type b is a problem in Developing Countries?
Epidemiology
General History
The advantages that well developed, industrialized nations have in combating infectious diseases is major contributing factor to the disparity in disease prevention for less developed countries. Especially in developing countries, Hib is most often diagnosed only by the diseases it causes, which are bacterial meningitis, S. pneumonia and, less commonly, Epiglottitus. Also, before the introduction of vaccines in 1990, Haemophilus influenzae type b (Hib) was responsible for the majority of the severe cases of more than 95% of invasive H.influenzae. disease among children younger than five years old. Meningitis was the leading cause in two-thirds of children with invasive disease, resulting in hearing impairment or severe permanent neurological consequences, such as retardation, seizure disorder, cognitive and developmental delay, and paralysis in 15%-30% of survivors. [8,5,7] The success of Hib polysaccharide vaccines in the 90’s followed by conjugate vaccines in 2000 clearly established that HiB was a preventable disease.[8] In fact, data cited from the World Health Organization in April of 2000 showed a 78% drop in Hib cases in children 0-4 yrs. in affluent countries since the advent of Hib conjugate vaccines. This is startling when compared with the worldwide statistic of only 5.9% in children of the same age group living in developing countries.[9] Accessibility to vaccines in third world countries is just one of many problems contributing to the spread of H. influenza. Antibiotic treatment in these countries comes with its problems as well. Most notably, the high cost of an extended duration of treatment and concern for antibiotic resistance.[9] But to truly understand why a preventable disease such as Hib is still a leading cause of pediatric mortality in developing countries, one must understand the basic requirements for disease prevention for any given country.[9]
Obstacles to Success and Prevention:
Insufficient Training and Lack of Technology
In a December 2005 media release from the World Health Organization it was stated “The two major obstacles to prevention of Hib disease are a shortage of information and a shortage of money”. [10] The WHO and many local government-sponsored Hib surveillance sites in third world regions, such as those in East Africa and Latin America, are the first point of contact for data and observations. It is in this patient contact that the physicians play a crucial role in reporting and diagnosing the disease. In an environment lacking in the required technology and few properly trained support staff, it is very difficult to get physicians to adopt WHO clinical protocols that require technology and human resources not readily available. Training in modern clinical management is also uncommon. Moreover, it is very rare to find a clinical physician in countries such as Uganda or Ecuador that specializes in pediatric disease which leads to a high incidence of misdiagnosis. This is understandable given that specialty training, such as pediatrics, is not a significant part of undergraduate curriculum for doctors trained in developing countries.[11]
Social and Cultural Obstacles
There are also social and cultural obstacles presented by physicians in developing countries as shown in a very valuable report on netSpear (The network for Surveillance of Pneumococcal Disease in the East African Region). It was observed that, in addition to inconsistent standards in laboratory operating procedures, physicians often only performed test when they thought it was beneficial to the child which contributed to a lack of quality data The report seems to infer that inadequate human recourses played a part in those types of decisions.[8] Given the similarity of the symptoms presented by Hib with that of the common cold, a misdiagnosed Hib infection can often be treated with toxic remedies indigenous to the local culture.[11]
Antibiotic Resistance
Although a major problem, inadequate clinical practices is of less concern than the emergence of an antibiotic resistant strain of Hib. Dr. Henry Campbell, a leading pediatric disease research scientist stated “The threat of penicillin and co-trimoxazole resistance is perhaps the most serious threat to the success of the WHO strategy of improved clinical management. The establishment of routine surveillance mechanisms to identify bacterial drug resistance in developing countries is an enormous challenge”. [11]
Barriers to Affordability
Although considerably less cost than antibiotics, accessibility to affordable conjugate (combination) vaccines in developing countries is by far the greatest obstacle. Aside from the cost related to technical difficulties with the physical delivery of vaccine to areas with little to no municipal infrastructure, it is the lack of financial incentive for many developing country governments to fund budgets for ongoing programs. Given there is inadequate surveillance, the data needed to reveal these incentives goes unseen. Government officials simply do not have access to the data that would be needed for adequately funding a sustainable immunization program. A Feb. 2003 bulletin from the World Health Organization stated one of the major causes of persistently low regional vaccination coverage was “Inadequately and un-sustained funding which have direct implication on service delivery operations such as outreach services, supervision and vaccine procurement”.[12] An adequate health surveillance system not only provides the information needed to combat Hib at the individual patient level, but it also provides the information needed to create the funding for the ongoing disease prevention programs for the entire developing region. This is true for both government funding and outside private investors. Both need large volumes of reliable information to base their financial decisions.[12]
What is being done to address these Problems in the Developing Countries?
Despite the existing prevention to this disease by giving immunizations at a young age, Hib vaccine is still not widely used in developing countries, where the greatest rates of Hib-related disease and deaths occur.[13]
Vaccine Initiatives
One of the well informative websites, “Hib Initiaitive,” is able to advertise information about the disease such as causes, effects, preventions and immunizations. The website aims to guide countries in forming decisions regarding introduction or continuation of Hib vaccine programs in the context of other health problems and offers technical assistance and support in the following areas: 1) research and surveillance, 2) planning coordination in decision making and implementation, 3) and advocacy and communication support for GAVI-eligible countries. The Hib Initiative works with GAVI-eligible countries around the world to support decisions regarding Hib vaccine introduction and sustainable implementation into immunization programs. [12] They also unite experts from Johns Hopkins Bloomberg School of Public Health, the London School of Hygiene and Tropical Medicine, the World Health Organization, and the Centers for Disease Control and Prevention (CDC). [14]
Vaccine Application
Since 1999, Global Alliance for Vaccines and Immunization (GAVI) through the Vaccine Fund has provided a mechanism of financing Hib conjugate vaccines for 5 years to the 75 poorest countries. GAVI allows eligible countries to purchase the vaccine for a 15 to 30 cents co-payment through 2015, which mostly are African and Asian countries. [15] In fact, South Africa was the first country in Africa to self-finance and incorporate Hib vaccine into its routine child immunization schedule since July 1999. It was also able to establish a national laboratory-based surveillance for invasive Hib disease to document the impact of routine vaccination of Hib disease. [16] Many middle income countries have now been able to adopt Hib-containing vaccines. Other, particularly lower-middle income countries, are struggling to find solutions as price is viewed as unaffordable. As more countries move towards adoption of more generic forms of vaccines, the market demand becomes more predictable and additional suppliers enter the market, and prices may begin to decline. As a result, one of the developing countries has stepped forward to test the benefits of this program.[15]
Example of a Success
Kenya, is an example of one country that has been taking advantage of the support from GAVI. Kenya being one of the first countries to receive this support was aided with over $67.4 million US, over the span of 5 years. When support from GAVI was about to cease, Kenya agreed to co-finance these vaccines with a total contribution of US $0.38 per dose from 2006 until 2011. Taking on this responsibility, the Kenyan government has to be constantly evaluate if this is a key issue deserving priority and governmental funds. A method that the World Health Organization advocates to deem whether something is cost-effective is to compare the Disability adjusted life years [DALY], which has been taken from the global burden of disease study, and compares it with that particular countries' GDP. In Kenya’s case, this value is significantly less than their GDP signifying that it is indeed cost-effective.[23]
Generic Forms Lower Vaccine Costs
Currently, there are large number of vaccine manufacturers that have Hib-containing vaccines both available and in development. For GAVI-eligible countries, there are suppliers that produce different generic formulations including a liquid/lyophilized vaccine and an all-liquid vaccine. The UNICEF supply division maintains current lists of available UNICEF-procured vaccines on their website and contains information on how the products are supplied, storage space required and price. As of January 2009, average prices range from $3.41 to $3.50 per dose. Average prices are expected to change and these changes will be announced by UNICEF.[15]
Assessment Tools
Another measure that has been done to properly evaluate the pervasiveness of the Hib disease in developing countries and to increase overall awareness to improve the situation is an assessment tool for Hib that is quick and easy to perform .[17] Certain steps have been made to help diagnose the people in these countries so they are more readily aware of the prevalence and severity in that particular country. Population-based laboratory procedures such taking spinal taps from children’s lumbar region and testing it have been a commonly used method because of its rapidity. Country-wide rates of Hib meningitis and pneumonia were accomplished in as little as 7 to 10 days through these quick assessments. These assessments link two processes together to predict the Hib rates. Combining estimates of Hib rates in the locality and the amount of children per 1,000 live births that die before their 5th birthday (under-5 mortality rate), the country is provided with a fast and very inexpensive way to assess their country’s Hib rate and the need for implementation of the vaccine. This has been extremely valuable, as the countries that were studied Ghana, Uganda, and Omen have requested the vaccinations from GAVI after these evaluations were performed.[13]
References
1)<http://www.who.int/immunization_monitoring/burden/EID_Hib_Jul04.pdf> Daniel R. Feikin,* Christopher B. Nelson,† James P. Watt,*‡ Ezzeddine Mohsni,§ Jay D. Wenger,† and Orin S. Levine*‡. Rapid Assessment Tool forHaemophilus influenzae typebDisease in Developing Countries1. Vol. 10, No. 7, July 2004. Emerging Infectious Diseases • www.cdc.gov/eid.
2)<http://www.healthsystem.virginia.edu/uvahealth/peds_infectious/hii.cfm>. Infectious Diseases , Haemophilus influenzae Infections.
3)<http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/hib-508.pdf>. Haemophilus influenzae type b.
4)<http://www.textbookofbacteriology.net/haemophilus.html>. Todar, Kenneth,PhD. Todar’s online Textbook of Bacteriology. Haemophilus influenzae and Hib Meningitis.
5)<http://www.cdc.gov/vaccines/pubs/surv-manual/chpt02-hib.pdf>. Kashif Iqbal, MPH; Leonard Mayer, PhD; Pamela Srivastava, MS; Nancy Rosenstein Messonnier, MD, MPH; Kristine M. Bisgard, DVM, MPH. Chapter 2: Haemophilus influenzaeType b Invasive Disease. VPD Surveillance Manual, 4th Edition, 2008.
6)<http://emedicine.medscape.com/article/218271-overview>. Vidya R Devarajan, MD, Haemophilu iInfluenzae Infections.
7)<http://www.paho.org/English/HVP/HVI/hvp_hib_epidprev.htm>. Haemophilus influenzae type b: Epidemiology and Prevention
8)<http://www.pubmedcentral.nih.gov/articlerender>. Sarah Mudhume et. Al. , Clinical Infectious Disease . 2009 March 1: 48(Suppl 2): S147-S152
9)<http://www.pubmedcentral.nih.gov/articlerender>. Peltola Heiki , Clinical Microbiology Reviews 2000 April: 13(2): 302-317.
10)<http://www.who.int/mediacentre/factsheets/>. World Health Organization(WHO). Media Centre Web page. Available at:. Accessed 20 August 2009.
11)<http://www.pubmedcentral.nih.gov/pagerender>. Campbell Harry . Archives of Disease in Childhood 1995: 73: 281-286 .
12)<http://www.who.int.org>. World Health Organization(WHO)AFRO EPI-Newsletter. Vaccines Preventable Disease Bulletin. Issue No. 32, February 2003.
13)<http://www.cdc.gov/ncidod/EID/vol10no7/03-0737.htm>. Feikin DR, Nelson CB, Watt JP, Mohsni E, Wenger JD, Levine OS. "Rapid assessment tool for Haemophilus influenzae type b disease in developing countries." 21 July 2004. Emerging Infectious Disease. 25 Aug 2009.
14<http://www.gavialliance.org/media_centre/press_releases/2009_08_11_india_pentavalent.php>.GAVI. GAVI Alliance / Pentavalent / India. 11 Aug 2009. 25 Aug 2009.
15)<http://hibaction.org>. Privor-Dumm, Lois and Hajjeh Rana. "Hib Initiative: Vaccine Supply & Finance." The Hib Initiative: Taking action to prevent childhood pneumonia and meningitis. 25 August 2009.
16)Von Gottberg, A., et al. "Impact of conjugate Haemophilus influenzae type b (Hib) vaccine introduction in South Africa." Bulletin of the World Health Association Oct 2006: 881-818.
17)<http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD001729/frame.html>. Swingler, George, Michaels, Desiree, Hussey Gregory G.D. “Conjugate vaccines for preventing Haemophilus influenzae type B infections.”
18)Campos-Outcalt, Douglas. 20 Common Problems in Preventative Health Care. McGraw-Hill, 2000. 13-14.
19)<http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2647418>. Lewis, Rosamund, et al. “Action for child survival: elimination of Haemophilus influenzae type b meningitis in Uganda.”
20)<http://www.brown.edu/Courses/Bio_160/Projects1999/bmenin/haeinfl.html>.
21)<http://www.cdc.gov/vaccines/vpd-vac/hib/photos.htm>.
22)<http://www2a.cdc.gov/nip/isd/ycts/mod1/courses/hib/10205.asp?student_id>.
23)Akumua, Angela O., et al. "Economic Evaluation of Delivering Haemophilus Influenzae Type b Vaccine in Routine Immunization Services in Kenya." Bulletin of the World Health Organization. Volume 85, No.7. July 2007. pg 501-568.
Edited by Nermin Salih, Frank Fu, Timothy Riley, Leslie Punzalan, students of Rachel Larsen