Dietzia cinnamea: Difference between revisions
| Line 55: | Line 55: | ||
Gerday, C., & Glansdorff, N. (2007). Physiology and biochemistry of extremophiles. Washington, D.C.: American Society for Mircrobiology Press. | Gerday, C., & Glansdorff, N. (2007). Physiology and biochemistry of extremophiles. Washington, D.C.: American Society for Mircrobiology Press. | ||
Iwaki, H., Nakai, E., Nakamura, S., Hasegawa, Y. Isolation and Characterization of Cyclohexylacetic Acid-degrading Bacteria. Current Microbiology. 2008. 57:107-110. | |||
Prescott, L., Klein, D., & Harley, J. (2002). Microbiology. Retrieved from Online Learning Center: http://highered.mcgraw-hill.com/sites/0072320419/student_view0/glossary_s-z.html | Prescott, L., Klein, D., & Harley, J. (2002). Microbiology. Retrieved from Online Learning Center: http://highered.mcgraw-hill.com/sites/0072320419/student_view0/glossary_s-z.html | ||
Revision as of 21:24, 23 April 2011
Classification
Domain: Bacteria
Phylum: Actinobacteria
Class: Actinobacteria
Order: Actinomycetales
Sub-order: Corynebacterineae
Family: Dietziaceae
Genus: Dietzia
NCBI Taxonomy ID:[1]
Species: Dietzia cinnamea
Description and Significance
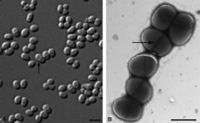
Samples of this microbe have been found in nature twice. The first was from a perianal swab from a patient with a bone marrow transplant (Yassin, 2006). The second, named P4, was extracted from petroleum contaminated soil characterized in acidic sandy loam Cambisol soil in a protected habitat in Rio de Janeiro state, Brazil (Von der Weid, 2006). Multiple strains of Dietzia have been found in soil, deep sea sediment, and soda lakes (Gerday & Glansdorff, 2007).Dietzia cinnamea is rod shaped in the medical swab while the P4 strain from the soil samples produces a coccoid shape. The organism is approximately 1.4 micrometers long, forms smooth, yellow to orange colonies on agar plates once isolated and is single or arranges in small connected colonies (Von der Weid, 2006). It is gram positive and has a high G+C content, meaning a high number of Guanine and Cytosine linkages in its DNA(Von der Weid, 2006). It displays snapping division, which is the arrangement of cells in a palisade or angular manor after binary fission (Prescott, 2002). This is a characteristic of the genera Arthrobacter and Corynebacterium.
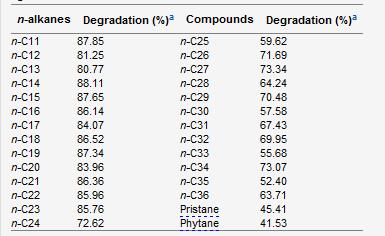
D. cinnamea is capable of degrading a range of petroleum hydrocarbons which can have beneficial environmental implications in today’s world. Other genera that have hydrocarbon degrading strains include Mycobacterium, Rhodococcus, and Dietzia. The strain P4 is able to degrade a range of n-alkanes (C11-C36), pristane, and phytane and is able to grow in the presence of carbazole, quinoline, naphthalene, toluene, gasoline, and diesel (Von der Weid, 2006).

Genome Structure
The full DNA code for D.cinnamea has been partially sequenced and contains 3,555,295 bp (NCBI Nucleotide):[2]. D.cinnamea has a GC content of 72.3% (Von der Weid, 2006). DNA of the P4 strain was sequenced with a 16S rDNA and it was found to have a 99.8% similarity to D.cinnamea that was found in the previous bone marrow transplant swab. When testing DNA-DNA similarities, the strains were 93.3% homologous with the transplant swab. A 70% threshold of DNA-DNA similarity is required to include it in the species. This confirms that P4 is part of the species D.cinnamea. This species has a 30-50% genome similarity with the other species of Dietzia (D.maris 52.4%, D.natronolimnaea' 56.3%, D.psychraicliphila 37.8%)(Von der Weid, 2006).
Cell Structure, Metabolism, and Life Cycle
Cell Structure
D.cinnamea is gram positive and ranges from rod to coccoid shaped. Average cell size is approximately 1.4 micrometers.
Metabolism
D.cinnamea is an aerobic organotroph that can utilize acetate, D-glucose, maltose, and 1,2 propandediol as a carbon source (Yassin, 2006). P4 uses maltose as a carbon source in addition to petroleum hydrocarbons including n-alkanes, toluene, diesel, gasoline, naphthalene, quinoline, and carbazole(Von der Weid, 2006). Successful growth was achieved when the petroleum hydrocarbons were used with mineral liquid media. D.cinnamea is therefore a useful microbe in bioremediation given its potential break down of petroleum based hydrocarbons. Specific mechanisms of hydrocarbon bioremediation is not fully understood currently (XX) Metabolic activities reduce nitrate to nitrite (Von der Weid, 2006).
Ecology and Pathogenesis
Dietzia has been found in multiple habitats including soil, deep sea sediment, and soda lakes.
References
Gerday, C., & Glansdorff, N. (2007). Physiology and biochemistry of extremophiles. Washington, D.C.: American Society for Mircrobiology Press.
Iwaki, H., Nakai, E., Nakamura, S., Hasegawa, Y. Isolation and Characterization of Cyclohexylacetic Acid-degrading Bacteria. Current Microbiology. 2008. 57:107-110.
Prescott, L., Klein, D., & Harley, J. (2002). Microbiology. Retrieved from Online Learning Center: http://highered.mcgraw-hill.com/sites/0072320419/student_view0/glossary_s-z.html
Von Der Weid, I., Marques, J. M., Cunha, C. D., Lippi, R. K., Dos Santos, S. C. C., Rosado, A. S., Lins, U., et al. (2007). Identification and biodegradation potential of a novel strain of Dietzia cinnamea isolated from a petroleum-contaminated tropical soil. Systematic and applied microbiology, 30(4), 331-339. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1717450
Yassin, A., Hupfer, H., & Schaal, K. (2006). Dietzia cinnamea sp.nov., a novel species isolated from a perianal swab of a patient with a bone marrow transplant. International Journal of Systematic and Evolutionary Microbiology , 641-645.
Authors
This page was created by Jennifer Jury and Elizabeth Karinen, students of Prof. Jay Lennon's Microbial Ecology Class at MSU.