Acidithiobacillus ferrooxidans: Difference between revisions
| Line 29: | Line 29: | ||
==Ecology and Industrial Applications== | ==Ecology and Industrial Applications== | ||
Interestingly, many isolations of ''A. ferrooxidans'' using purely inorganic media | ==Ecology and Industrial Applications== | ||
Interestingly, many isolations of ''A. ferrooxidans'' using purely inorganic media that were thought to be pure cultures, were actually contaminated with members of the genus ''Acidiphilium''. This prompted scientists to study a possible symbiosis between the two organisms. ''Acidiphilium'' was believed to aide ''A. ferrooxidans'' growth in unfavorable conditions by metabolizing organic materials. It is well documented that ''A. ferrooxidans'' is notably intolerant of low molecular weight organic acids. Cultivation of ''A. ferrooxidans'' will be very poor on acidic ferrous iron agar because growth becomes inhibited by agar hydrolyzed products like: pyruvic acid, citric acid, oxaloacetic acid and glucose. Members of the genus ''Acidiphilium'' are oligotrophs, liking very low concentrations of organic compounds in growth media. Common heterotrophic media (eutrophic) is too rich in glucose and other organic compounds for ''Acidiphilium cryptum'' to grow at all. This is the reason that these two microbes grow so well in co-culture. The ''Acidiphilia'' scavenge for the organic compounds excreted by ''A. ferrooxidans'' such as: pyruvate, glutamate, aspartate, serine, and glycine. Additionally, some members of the genus ''Acidiphilium'' are capable of iron reduction, which is the opposite process performed by ''A. ferrooxidans''. By removing the organic compounds that harm ''A. ferrooxidans'', oxidation of ferrious iron will be favorable. Lastly, a common oxidation product of pyrite (FeS<sub>2</sub>), thiosulfate, is very toxic to ''Acidiphilium''. ''A. ferrooxidans'' can oxidize thiosulfate preventing damage to occur to ``Acidiphilium’’. In addition, thiosulfate is very unstable and breaks down in acidic conations, another by-product of ''A. ferrooxidans'' growth. | |||
There are many ways in which ''Acidithiobacillus ferrooxidans'' can be used for industrial purposes. Most of these have to do with a process called bioleaching or biohydrometallurgy. Essentially, this proteobacteria is so useful because it is able to attack sulfide containing minerals and can convert insoluble sulfides of metals like copper, lead, zinc and nickel to their respective soluble metal sulfates. Bioleaching involves using micrbial communities to extract desirable metals from unrefined ores. It works especially well for low grade ores. Environmental impact is also reduced compared to traditional concentration and smelting techniques. Another important microbial-mining process is known as desulfurization. Commonly coal can have undesirable sulfur compounds created by natural weathering processes. This undesirable sulfur can be solublized by ''A. ferrooxidans'' and removed by washing techniques. Furthermore, these techniques have shown significant promise in a few other areas. During the recycling of computers and electronics a lot of dust-like materials is created through shredding and separation. These dusty residues are known to contain valuable concentrations of metals that would otherwise be incinerated or thrown in a landfill. In one experiment, over 90% of the available Cu, Zn, Ni and Al was leached out of this computer circuit board dust. | There are many ways in which ''Acidithiobacillus ferrooxidans'' can be used for industrial purposes. Most of these have to do with a process called bioleaching or biohydrometallurgy. Essentially, this proteobacteria is so useful because it is able to attack sulfide containing minerals and can convert insoluble sulfides of metals like copper, lead, zinc and nickel to their respective soluble metal sulfates. Bioleaching involves using micrbial communities to extract desirable metals from unrefined ores. It works especially well for low grade ores. Environmental impact is also reduced compared to traditional concentration and smelting techniques. Another important microbial-mining process is known as desulfurization. Commonly coal can have undesirable sulfur compounds created by natural weathering processes. This undesirable sulfur can be solublized by ''A. ferrooxidans'' and removed by washing techniques. Furthermore, these techniques have shown significant promise in a few other areas. During the recycling of computers and electronics a lot of dust-like materials is created through shredding and separation. These dusty residues are known to contain valuable concentrations of metals that would otherwise be incinerated or thrown in a landfill. In one experiment, over 90% of the available Cu, Zn, Ni and Al was leached out of this computer circuit board dust. | ||
Revision as of 15:26, 24 April 2011
Classification
Bacteria; Proteobacteria; Gammaproteobacteria; Acidithiobacillales; Acidithiobacillaceae
Species
Acidithiobacillus ferrooxidans PubMed Genome of ATCC 23270 Genome of ATCC 53993
Description and Significance
A. ferroxidans is a Gram negative rod shaped bacterium that is commonly found in deep caves or acid mine drainage, such as coal waste (10, 11, 12). These acidophilic bacteria thrive in optimal pH level of 1.5 – 2.5 where they are able to convert insoluble heavy metals to their soluble state. Even low concentrations (ppm) of these metallic ions would be extremely toxic to other bacteria (7, 8). These bacteria have been utilized in industrial bioleaching efforts to extract otherwise unobtainable metals (9).
Genome Structure
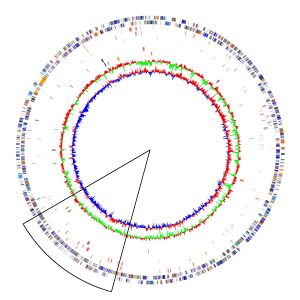
Two strains of Acidthiobacillus ferrooxidans have been successfully and completely sequenced. The strains sequenced are ATCC 53993 and ATCC 23270 which consists of genomic sizes of 2.88kb and 2.98kb respectively. Acidithiobacillus ferrooxidans contains a single circular chromosome consisting of around 3,000 genes. The greater majority of species sampled from multiple sites around the world were shown to contain plasmids, however ATCC 23270 did not contain a plasmid (15). ATCC 23270 contains a rich region of recombination which includes phage, transposases, and psuedogenes shown in the black triangle located in Figure 1.
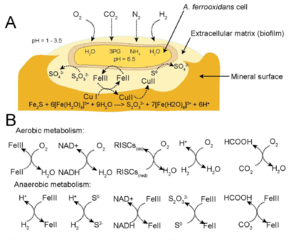
Cell Structure and Metabolism
A. ferrooxidans is a chemolithotrophic bacterium which can use many different electron donors to support growth. In addition, a primary habitat consists of acidic conditions producing a strong reducing environment. The cell is able to maintain homeostasis with a neutral pH of 6.5 within the cytoplasm preventing damage and a reducing environment in periplasm. This allows an increase in redox potential of O2/H2O to 1.12 V at pH 3.2 yielding higher energy when coupled with an electron donor (13, 14).
In aerobic conditions, electron donors may include ferrous ions or sulfur compounds which are oxidized into ferric iron and sulfuric acid, respectively, yielding high energy (3, 4, 5). However, during anaerobic conditions ferric ions can replace oxygen as the electron acceptor with multiple substrates donating an electron (Figure 2B, 6). This pathway yields less energy than aerobic conditions, but energy can still be produced for growth.
A. ferrooxidans can fix atmospheric carbon dioxide (CO2) as a carbon source essential for biomass (1). Nitrogen is a common limiting nutrient scarce in the environment; however, A. ferrooxidans can fix atmospheric nitrogen into ammonia (NH3) essential for nucleotides and amino acids (2).
Ecology and Industrial Applications
Ecology and Industrial Applications
Interestingly, many isolations of A. ferrooxidans using purely inorganic media that were thought to be pure cultures, were actually contaminated with members of the genus Acidiphilium. This prompted scientists to study a possible symbiosis between the two organisms. Acidiphilium was believed to aide A. ferrooxidans growth in unfavorable conditions by metabolizing organic materials. It is well documented that A. ferrooxidans is notably intolerant of low molecular weight organic acids. Cultivation of A. ferrooxidans will be very poor on acidic ferrous iron agar because growth becomes inhibited by agar hydrolyzed products like: pyruvic acid, citric acid, oxaloacetic acid and glucose. Members of the genus Acidiphilium are oligotrophs, liking very low concentrations of organic compounds in growth media. Common heterotrophic media (eutrophic) is too rich in glucose and other organic compounds for Acidiphilium cryptum to grow at all. This is the reason that these two microbes grow so well in co-culture. The Acidiphilia scavenge for the organic compounds excreted by A. ferrooxidans such as: pyruvate, glutamate, aspartate, serine, and glycine. Additionally, some members of the genus Acidiphilium are capable of iron reduction, which is the opposite process performed by A. ferrooxidans. By removing the organic compounds that harm A. ferrooxidans, oxidation of ferrious iron will be favorable. Lastly, a common oxidation product of pyrite (FeS2), thiosulfate, is very toxic to Acidiphilium. A. ferrooxidans can oxidize thiosulfate preventing damage to occur to ``Acidiphilium’’. In addition, thiosulfate is very unstable and breaks down in acidic conations, another by-product of A. ferrooxidans growth.
There are many ways in which Acidithiobacillus ferrooxidans can be used for industrial purposes. Most of these have to do with a process called bioleaching or biohydrometallurgy. Essentially, this proteobacteria is so useful because it is able to attack sulfide containing minerals and can convert insoluble sulfides of metals like copper, lead, zinc and nickel to their respective soluble metal sulfates. Bioleaching involves using micrbial communities to extract desirable metals from unrefined ores. It works especially well for low grade ores. Environmental impact is also reduced compared to traditional concentration and smelting techniques. Another important microbial-mining process is known as desulfurization. Commonly coal can have undesirable sulfur compounds created by natural weathering processes. This undesirable sulfur can be solublized by A. ferrooxidans and removed by washing techniques. Furthermore, these techniques have shown significant promise in a few other areas. During the recycling of computers and electronics a lot of dust-like materials is created through shredding and separation. These dusty residues are known to contain valuable concentrations of metals that would otherwise be incinerated or thrown in a landfill. In one experiment, over 90% of the available Cu, Zn, Ni and Al was leached out of this computer circuit board dust.
Detection of Acidithiobacillus ferrooxidans in acid mine drainage
environments using fluorescent in situ hybridization (FISH)
Essential interactions between Thiobacillus ferrooxidans and
heterotrophic microorganisms during a wastewater sludge
bioleaching process
Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi
Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture
Bioleaching and mineral biotechnology
Influence of heavy metals on growth and ferrous sulphate oxidation by Acidithiobacillus ferrooxidans in pure and mixed cultures
The role of Acidithiobacillus ferrooxidans in alleviating the inhibitory effect of thiosulfate on the growth of acidophilic Acidiphilium species isolated from acid mine drainage samples from Garubathan, India
References
1. Kelly, D. P., and A. P. Harrison. 1989. Genus Thiobacillus Beijerinck, p. 1842-1858. In J. T. Staley, M. P. Bryant, N. Pfennig, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 3. The Williams & Wilkins Co., Baltimore.
2. Mackintosh, M. E. 1978. Nitrogen fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 105:215-218.
3. Pronk, J. T., K. Liem, P. Bos, and J. G. Kuenen. 1991. Energy transduction by anaerobic ferric iron respiration in Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 57:2063-2068.
4. Corbet, C. M., and W. J. Ingledew. 1987. Is Fe3+'2+ cycling an intermediate in sulphur oxidation by Fe2+-grown Thiobacillus ferrooxidans? FEMS Microbiol. Lett. 41:1-6.
5. Sugio, T., K. J. White, E. Shute, D. Choate, and R. C. Blake. 1992. Existence of a hydrogen sulfide:ferric ion oxidoreductase in iron-oxidizing bacteria. Appl. Environ. Microbiol. 58:431-433.
6. Sugio, T., C. Domatsu, 0. Munakata, T. Tano, and K. Imai. 1985.
7. Role of a ferric ion-reducing system in sulfur oxidation of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 49:1401-1406.
8. Rawlings, D. E., I.-M. Pretorius, and D. R. Woods. 1986. Expression of Thiobacillus ferrooxidans plasmid functions and the development of genetic systems for the thiobacilli. Biotechnol. Bioeng. Symp. 16:281-287.
9. Colmer, A. R., and M. E. Hinkle. 1947. The role of microorganisms in acid mine drainage: a preliminary report. Science 106:253–256.
10. Yu Yang, Min-xi Wan, Wu-yang Shi, Hong Peng, Guan-zhou Qiu, Ji-zhong Zhou, Xue-duan Liu. 2007. Bacterial diversity and community structure in acid mine drainage from Dabaoshan Mine, China. AQUATIC MICROBIAL ECOLOGY Vol. 47: 141-151
11. Brierley, C. L. 1982. Microbiological mining. Sci. Am. 247(2):42-51.
12. Merson, J. 1992. Mining with microbes. New Sci. 133:17-19.
13. Sato, A., Y. Fukumori, T. Yano, M. Kai, and T. Yamanaka. 1989. Thiobacillus ferrooxidans cytochrome c-552: purification and some of its molecular features. Biochim. Biophys. Acta 976:129-134.
14. Cox, J. C., and M. D. Brand. 1984. Iron oxidation and energy conservation in the chemoautotroph Thiobacillus ferrooxidans, p.31-46. In W. R. Strohl and 0. H. Touvinen (ed.), Microbial chemoautotrophy. Ohio State University Press, Columbus, Ohio.
15. Valdés, J., et. al. 2008. Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications, BMC Genomics 9:597