Mycobacteriosis (Fish Tuberculosis): Difference between revisions
| Line 33: | Line 33: | ||
One factor that has led to a greater portion of the human population is the survival of CPM outside of the host (the fish)[1]. The CPM can be found in the water, on other organisms, on plant matter, and on abiotic substances (See Figure 3). The location or environment of the mycobacteria does not play as much of a role in the survival of any of the species as much as the temperature. CPM grow ideally above 18-20 ̊C, but also survive below these temperatures for months. Environmental infestations have proved to cause many of the cases of disease transfer to humans. The disease can be transferred by the touching or ingesting of any infected object or organism. The mycobacteria have even been found on clinically healthy fish at low concentrations, but it is unclear whether this concentration has the ability to infect humans or not. | One factor that has led to a greater portion of the human population is the survival of CPM outside of the host (the fish)[1]. The CPM can be found in the water, on other organisms, on plant matter, and on abiotic substances (See Figure 3). The location or environment of the mycobacteria does not play as much of a role in the survival of any of the species as much as the temperature. CPM grow ideally above 18-20 ̊C, but also survive below these temperatures for months. Environmental infestations have proved to cause many of the cases of disease transfer to humans. The disease can be transferred by the touching or ingesting of any infected object or organism. The mycobacteria have even been found on clinically healthy fish at low concentrations, but it is unclear whether this concentration has the ability to infect humans or not. | ||
The concern with the range of substances mycobacteria inhabit has to do with the fact that water used for drinking and facilities have been contaminated in the past with infectious species.<br> | The concern with the range of substances mycobacteria inhabit has to do with the fact that water used for drinking and facilities have been contaminated in the past with infectious species [3].<br> | ||
==Pathogenic Mechanism== | ==Pathogenic Mechanism== | ||
Revision as of 18:05, 11 May 2011
Introduction
By Sally Moseley
Mycobacteria are a class of rod-shaped bacteria that are infamous for the difficulty they pose in detecting and isolating the cells within their host organisms. This is due to the fact that mycobacteria (including Myobacterium marinum), are acid-fast. For these species, procedures such as Ziiehl-Neelsen staining and biopsy culture are necessary for detection instead of using traditional means of staining and examining. The organism is difficult to isolate by a normal swab procedure. For effective analysis of specific species of mycobacteria, polymerase chain reaction (PCR) for the 65-kDa protein (the protein with a highly conserved genetic code amongst the mycobacterial species, particularly notable between M. marinum and M. tuberculosis) and analysis the PCR products using restriction enzyme analysis are used [14]. These methods prove most effective with the touch cell type to isolate. Many products are analyzed using BACTEC technology, which detects microbial growth from blood specimens, which is useful in mycobacterial species that inhabit macrophages [14].
History
Mycobacterium marinum was the first species of pathogenic fish mycobacteria isolated in 1926 from dying and dead saltwater fish in the Philadelphia aquarium [5]. Years later, in 1951, it infected swimmers in Australia [8]. Cultures of mycobacteria were cultured from the lesions of the arms and legs of the swimmers. This was the first known case of the pathogen spreading to humans. It was realized immediately that the bacteria could not be grown at temperatures of 37 ̊C and above. The cultures did grow in 33 ̊C, and it was discovered later that temperatures between 25 and 35 ̊C were ideal for the bacteria.
In 1998, there were several accounts of an increase in M. chelonae in the Atlantic Ocean, particularly in the salmonid population [5]. This was a greater cause for concern in the 1950s when adding fish meal to the diet was more common and, even worse, more fish meal was unpasteurized. By 1998, pasteurization was becoming more common and the fish disease was not as much of an issue. There also appeared to be a connection between the mycobacterium infections and Vibrio sp. infections, but it may have been a coincidence due to the immunosuppressive symptoms of mycobacteriosis making the fish more susceptible to other conditions such as vibriosis. In these fish, mortality was caused by the lesions beginning with mycobacteriosis and not vibriosis.
Fish Tuberculosis
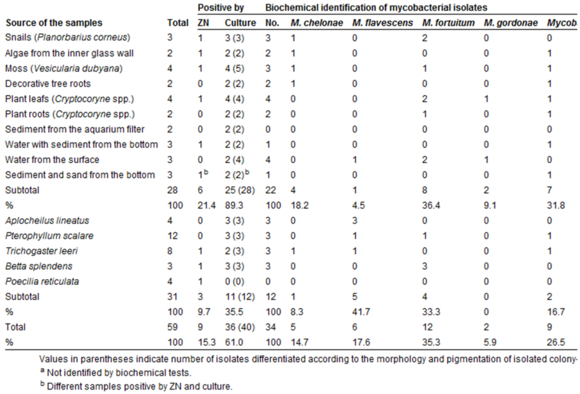
Several species of mycobacteria infect fish with fish tuberculosis, including M. fortuitum, M. flavescens, M. chelonae, M. gordonae, M. terrae, M. triviale, M. diernhoferi, M. celatum, M. kansasii ,M. intracellulare, and M. marinum. Because of the wide range of fish found to be infected with different Mycobacterium, it is assumed that all fish are susceptible to fish tuberculosis [6].
Different strains of mycobacteria are found worldwide and certain strains have been fairly ubiquitous around the world [3]. Tropical aquarium fish appear to be the most susceptible, though certain strains grow in the fish of other environments more readily. Because of the ideal temperature range (25-35 ̊C), mycobacteriosis is not as commonly found in fish in cold temperature zones. Like human tuberculosis, it is a progressive condition that may not show signs until years after the infection begins. In many fish, it is not apparent that they are infected until much later in the disease [6]. The first sign of infection are seen in abnormal growth on the liver and spleen. Later in the disease, typical signs can be seen externally in unusual wear on the scales and skin of the fish, which may include lesions. Infected species are often emaciated and lethargic.
Human Pathogenicity
M. fortuitum, M. chelonae and M. marinum, among others, are known to be zootonic and are therefore the subject of most studies for both health and economic reasons. The zootonic strains are conditionally pathogenic mycobacteria, or CPM [7]. M. marinum has proved to be the most prevalent cause of disease transfer. It is the primary pathogenic mycobacteria and earlier studies only mention M. fotuitum and M. chelonae as present in affected humans as opposed to the causative agent. Effects on the human population usually result in hand lesions from handling infected fish. The infection tends to stay at the extremities of the human body because of the cooler temperatures they provide as opposed to the internal temperatures. Fortunately, even though the bacterium prove fatal to fish, it usually only results in a cutaneous infection for humans [15].
These infections, however, can be chronic. Lesions tend to be noticed two to four weeks after exposure to the mycobacterium. They may be completely painless, irritating, or give discomfort. The lesions swell and develop into ulcerations, often leading to granuloma. In some instances, infection spreading to the lymphatics lead to a sporotrichoid pattern of infection. Some long-term effects of lesions are tenosynovitis, osteomyelitis, arthritis, and a compromised immune system [5][10]. The skin, kidney, and liver are the main body parts affected in both fish and humans. Fish tuberculosis is especially characterized by causing granulomatous in its host organism. When a granuloma is formed, the mycobacteria form epithelioid layers as they pack together. The nodules of each granuloma is usually covered by a thin capsule. The presence of mycobacteria is prolific; The pathogens can be found within cells and spread throughout the tissues of infected organs. When growing within eukaryotic cells, the replication of the bacteria, though slower than many pathogens, eventually reaches critical levels that kill the cells before lysing the membranes It appears that mycobacteria cells, both CPM cells and non-pathogenic mycobacteria, can be internalized by macrophages and epithelial cells readily, though mycobacteria has been found in several other cell types. Interestingly, pathogenic cells can be internalized by and survive and reproduce in HeLa cells, which is not normally the case for non-pathogenic cells.
The fish pathogen M. fortuitum has also been associated with masses found in cows and sheep, abscesses in dogs, granulomas in cats, and “spinning disease” (a neurological disorder) in mice, but the relation between these ailments is still unclear. M. fortuitum is particularly potent if the host organism already has an open would and is known to easily infect these wounds.
Pathogenic strains of different mycobacteria, though associated with different animals, have been known to infect a range of animals. In studying the pathogenicity of certain strains, the control mycobacteria used also ended up affecting the host organism. Usually, the newly pathogenic strain of mycobacteria is not as potent as the strain previously known to affect the particular host, but these experience describe the range with which mycobacterium can survive. Talaat et al. demonstrated that an environmental mycobacterium, M. smegmatis, (normally found in soil environments and found as the source of an infection in many wounds of several species of animals) caused more massive granulomatous in goldfish (Carassius auratus) than the known fish pathogen M. fortuitum.
One factor that has led to a greater portion of the human population is the survival of CPM outside of the host (the fish)[1]. The CPM can be found in the water, on other organisms, on plant matter, and on abiotic substances (See Figure 3). The location or environment of the mycobacteria does not play as much of a role in the survival of any of the species as much as the temperature. CPM grow ideally above 18-20 ̊C, but also survive below these temperatures for months. Environmental infestations have proved to cause many of the cases of disease transfer to humans. The disease can be transferred by the touching or ingesting of any infected object or organism. The mycobacteria have even been found on clinically healthy fish at low concentrations, but it is unclear whether this concentration has the ability to infect humans or not.
The concern with the range of substances mycobacteria inhabit has to do with the fact that water used for drinking and facilities have been contaminated in the past with infectious species [3].
Pathogenic Mechanism
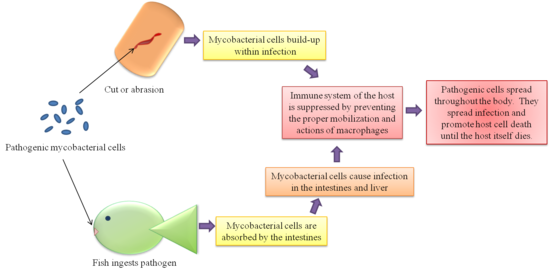
An interesting feature of M. marinum among other pathogenic mycobacteria is their survival in host macrophages [2][15]. Several theories have been proposed from various tests. As to why the mycobacteria survive assault by the host cell’s immune system. One theory is that the phagosomes within the macrophages containing the mycobacteria fail to bind the lysosomes that would destroy the mycobacteria cell. This may be because the phagosome with the mycobacteria is only mildly acidified and the lysosome does not recognize the mycobacteria as a threat. Another theory arriving from different studies suggests that the mycobacteria are able to break the membrane of the phagosome and free themselves into the cytoplasm of the macrophages. The former theory is more widely accepted, but there is still much debate about the actual mechanism of survival.
This former theory has been promoted by studies of vesicular proton ATP-ase, or V-ATPase [2]. V-ATPase is an enzyme located on the membranes of vesicles within macrophages that allows the vesicles containing foreign and unfavorable substances to be acidified and guides the vesicle to the lysosome that will digest the contents of the vesicle. Some studies suggest that the vesicles containing the mycobacteria do not have V-ATPase incorporated into their membrane. The mycobacteria may have a mechanism that repels them from the enzyme itself and enables them to take advantage of the fluid membrane and structurally change the membrane of host macrophage cells to exclude V-ATPase. Many studies have shown that vesicles containing mycobacteria have a low acidity and the studies of V-ATPase may be the key to this feature that prevents the death of the pathogen.
In one study, the exclusion of V-ATPase from the vesicle membrane containing the mycobacteria relies upon the livelihood of the mycobacteria themselves. The vesicles containing dead mycobacteria do not appear to have the same rate of V-ATPase exclusion and thus the host cells are able to eradicate them. This leads to the conclusion that the active metabolism and processes of the mycobacteria allow for their survival as opposed to membrane proteins that may have possibly played a role. Dead, UV treated mycobacteria, in which the cell wall has been compromised, are able to exclude V-ATPase from the first vesicle they enter into the host cell. This vesicle remains at a low acidity. However, the host cell eventually transfers the UV-treated mycobacteria into an acidified vesicle and dispose of the foreign materials. This suggests that the mycobacteria secrete a protein to exclude the V-ATPase regularly because the freshly dead cells would still contain the residue of this protein and exclude the V-ATPase for the first round. After the initial amount of this residue wears off, the mycobacteria are transferred to vesicles containing the V-ATPase. This would explain this mechanism and why the cells have to secrete the V-ATPase excluding substance continuously.
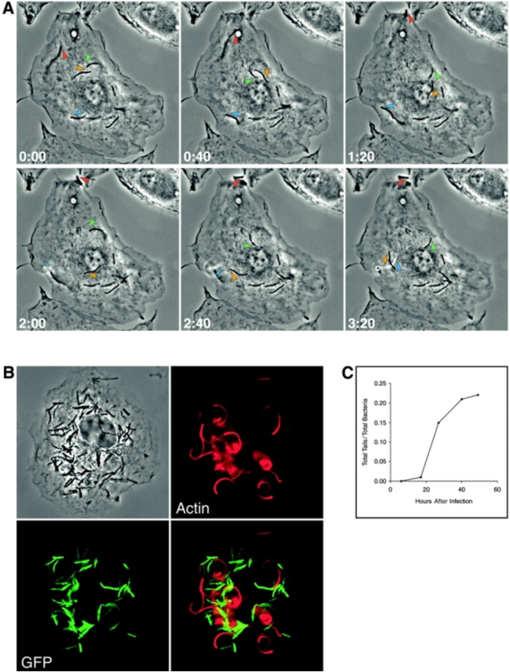
The mycobacterial-escape theory is supported by the discovery of mobile bacteria in more recent studies. M. marinum are motile when they reside in macrophage vesicles [2]. The movement is actin-based and propelled by actin tails that trail the mycobacteria as they travel. Similar to muscle movement, the actin tails is effective by polymerizing one and catabolizing the other end. This movement allows the bacteria to free themselves from the vesicle and escape into the cell cytoplasm. The movement does not occur if the mycobacteria are located outside of a host cell, but may provide a means for the mycobacteria to travel directly from one cell to another. This would be a procedure done in cases where the host cells are tightly packed together and the mycobacteria have easy access to the next adjacent cell.
M. marinum as a Study Tool
M. marinum is a useful tool in tuberculosis studies because of its close association with M. tuberculosis, the pathogen that causes tuberculosis and its relatively short generation time [2]. The generation time of M. tuberculosis is about twenty hours and the generation time of M. marinum is about four hours. The shorter generation time allows for further studies to work faster and be more productive. M. marinum also, even though it is closely associated to M. tuberculosis, does not have the potency of M. tuberculosis in humans. The pathogen tends to stay in the extremities of the body and there have been no reported cases of a breakdown in the organs of affected humans, even in cases of immunocompromised symptoms. This makes the handling of the bacteria easier
The growth rate of mycobacteria depends on the species. Even though M. marinum has a generation time of four hours, this is not true for all species of mycobacteria. Species of mycobacteria can be defined by their rate of isolation. Fast-growing mycobacteria take a couple of days to isolate and slow-growing mycobacteria take weeks or months to isolate [2].
Diagnosis
The diagnosis of fish tuberculosis infecting humans is difficult because the pathogens (particularly M. marinum) are able to mimic more common ailments, such as gout, rheumatoid arthritis, and chronic pyogenic infections [6]. Infections from M. marinum are clinically and histologically indistinguishable from tuberculosis. The combined mimicry with the rarity of fish-tank and swimming-pool granuloma often leads to faulty diagnosis when the patient is actually infected with mycobacteria. The distinctive qualities of mycobacteriosis are the patterns of the lesions (remaining near the cooler surface of human flesh as opposed to delving deeper in the body where it is warmer and less ideal for the bacteria to reside). Diagnosis is often helped by noting previous behavior, such as contact to certain waters or soils with the possibility of contamination with mycobacterial species.
Treatment
Treatment of mycobacteriosis in humans has proved difficult [6]. There are a number of antimicrobial products used to treat the skin condition, but no one combination of these antimicrobials appears to be universal. Rarely is one antimicrobial product enough to fully cure the condition of the patient, though cases of significant improvement have been recorded [7]. Other factors that vary widely case-by-case are antimicrobial dosage and timing (including the importance of starting treatment early and the timing in between dosages). In some cases, and effective cure was not even reported. The research of therapy methods is stunted by the fact that the condition is so rare. This prevents means of getting a comprehensive study with conclusive results. Some treatments include erythromycin, minocycline, doxycycline, trimethoprim-sulfamethoxazole, clarithromycin, ciprofloxacin, ethambutol, kanamycin, vitamin B6 rifabutin, and rifampicin. It may take weeks to over a year to find a suitable treatment or for a treatment to be fully effective. The current treatments have proved more difficult to obtain if the disease has developed the immunosuppressive symptoms. Fortunately, however, there have been no documented cases of any strains becoming resistant to antibiotics. The lack of a definitive or universal cure is not fully understood.
In deep lesions, surgery is often necessary. This is an undesirable action, however, because it is easy for the infection to spread to different parts of the body. It is very rare for mycobacteria to pose a serious threat to humans unless the human is already immunocompromised by diseases such as human immunodeficiency virus (HIV). In these cases, mycobacteriosis may prove fatal and individuals with compromised immune systems are advised against swimming in certain waters that have a higher possibility of being contaminated and drinking water that has not been through particular filters that prevent such infection. Patients with HIV or AIDS (acquired immune deficiency syndrome) and mycobacteriosis may go through transplant procedures of the spleen, liver, and kidney, but the mycobacteria still linger on the outside of the organs and the transplants fail to eradicate the pathogen completely.
BCG (M. bovis bacilli Calmette-Guerin) vaccination is a vaccine used to prevent tuberculosis . It has been shown that it may also be used against infection by M. marinum [2]. This is regarded as a good use of prevention, but also undermines the vaccine’s specificity. The vaccine may prevent other forms of mycobacteriosis at varying degrees. The vaccine has not been tested against M. marinum or other fish mycrobacteria on any human subjects. BCG is preventative and is not to be given to patients who have already contracted a form of mycobacteriosis.
Culturing Oddities
Naturally occurring mycobacteria has the confusing property of being more fatal than cultured lab mycobacteria. Though strains of mycobacteria can be cultured using MacConkey agar and blood agar plates, infecting fish species with the cultured bacteria infects less fish at a lower percentage with less to no chance of mortality [5]. There has been no explanation to why this may be. There is also some debate in whether mycobacteria grown in environmental biota and abiotic substances are as potent as mycobacteria grown within fish. This is a more difficult aspect to study because it is difficult to determine if mycobacteria has traveled from fish to the external environment or the other way around.
Recent Concerns
Since the first outbreak in 1997, the Chesapeake Bay area (including the tributaries leading to the Bay) near Maryland and Virginia has had a concerning number of fish (specifically, the striped bass, or Morone saxatilis) documented with mycobacteriosis and thus a fairly high number of humans affected with all three of the pathogenic strains of fish tuberculosis [5]. The Bay serves as a popular fishing spot. New warnings are being implemented for fishermen to wear gloves and extra cover on their bodies when fishing, a new feature of known mycobacteriosis-contaminated sites.
Conclusion
Mycobacterial studies are relevant in human health because of the CPM variations of fish tuberculosis and the relationship between fish tuberculosis and human tuberculosis. The mycobacterial species provide a way to study tuberculosis that is easier for human researchers to handle and more practical for the studies involved. This could help the development of a more successful vaccine against tuberculosis and may help to understand the disease’s debilitating properties better to prevent them from occurring in human tuberculosis.
Even though CPMs are rare, it is also important that the knowledge of its effects are spreading. Fifty years ago, mycobacteriosis was poorly understood and treatment based on symptoms was unsuccessful. With the spread of mycobacteriosis to more commercial environments, being aware of the dangers of the water is an important factor to prevent the discomfort that results from mycobacteriosis. Patients are often disabled by the lesions for weeks at a time. Economies and human health may rely on the further understanding of the spread and infection by mycobacterial species.
References
[16] Rainbowfish Keeping. http://rainbowfish.angfaqld.org.au/Myco.htm.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.