Ophiocordyceps sinensis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 65: | Line 65: | ||
(3) Wang XL and Yao YJ. "Host insect species of Ophiocordyceps sinensis: a review." ZooKeys, 2011, DOI: [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3175130/ 10.3897/zookeys.127.802] | (3) Wang XL and Yao YJ. "Host insect species of Ophiocordyceps sinensis: a review." ZooKeys, 2011, DOI: [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3175130/ 10.3897/zookeys.127.802] | ||
(4) Yang YX, Yang DR, Shen FR, and Dong DZ. "Studies on Hepialid larvae for being infected by Chinese "insect herb" fungus (Cordyceps sinensis) Zool Res, 1989, 10(3):227-231 [http://en.cnki.com.cn/Article_en/CJFDTOTAL-DWXY198903009.htm] | (4) Yang YX, Yang DR, Shen FR, and Dong DZ. "Studies on Hepialid larvae for being infected by Chinese "insect herb" fungus (Cordyceps sinensis)" Zool Res, 1989, 10(3):227-231 [http://en.cnki.com.cn/Article_en/CJFDTOTAL-DWXY198903009.htm] | ||
(5) Zhang YJ, Bai FB, Zhang S, and Liu XZ. "Determining novel molecular markers in the Chinese caterpilar fungus Ophiocordyceps sinensis by screening a shotgun genomic library." Appl Microbiol Biotechnol, 2012a, DOI 10.1007/s00253-012-4028-x | (5) Zhang YJ, Bai FB, Zhang S, and Liu XZ. "Determining novel molecular markers in the Chinese caterpilar fungus Ophiocordyceps sinensis by screening a shotgun genomic library." Appl Microbiol Biotechnol, 2012a, DOI [http://www.springerlink.com/content/j83650k36411xt77/ 10.1007/s00253-012-4028-x] | ||
(6) Zhang YJ, Li EW, Want CS, Li YL, and Liu XZ. "Ophiocordyceps sinensis, the flagship fungus of China: terminology, life strategy and ecology." Mycology, 2012b, | (6) Zhang YJ, Li EW, Want CS, Li YL, and Liu XZ. "Ophiocordyceps sinensis, the flagship fungus of China: terminology, life strategy and ecology." Mycology, 2012b, DOI [http://www.tandfonline.com/doi/abs/10.1080/21501203.2011.654354 10.1080/21501203.2011.654354] | ||
(7) Zhang YJ, Xu LL, Zhang S, Liu XZ, An ZQ, Wang M, and Guo YL. "Genetic diversity of Ophiocordyceps sinensis, a medicinal fungus endemic to the Tibetan Plateau: Implications for its evolution and conservation" BMC Evol Biol, 2009, 9:290 | (7) Zhang YJ, Xu LL, Zhang S, Liu XZ, An ZQ, Wang M, and Guo YL. "Genetic diversity of Ophiocordyceps sinensis, a medicinal fungus endemic to the Tibetan Plateau: Implications for its evolution and conservation" BMC Evol Biol, 2009, 9:290. DOI [http://www.biomedcentral.com/1471-2148/9/290/ 10.1186/1471-2148-9-290] | ||
===Other References=== | ===Other References=== | ||
Revision as of 05:38, 24 November 2012
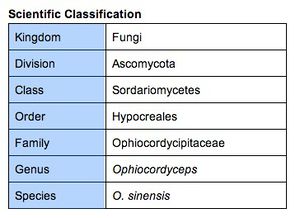
Classification and Naming
Traditionally, Ophiocordyceps sinensis was classified under the genus Cordyceps. The etymology of the scientific name is from Latin: cord “club”, ceps “head”, and sinensis “from China”. All species within the genus Cordyceps are endoparasitic; these species (entomopathogenic fungi) mainly parasitize insects and arthropods as well as other fungi. However, recent molecular analysis has determined that the Cordyceps genus is not monophyletic [14]. This resulted in the transfer of O. sinensis as well as several other Cordyceps species to the genus Ophiocordyceps.
O. sinensis is more commonly known as caterpillar fungus, and mistakenly referred to as “vegetable caterpillar”. The Chinese name, dong chong xia cao (冬蟲夏草) meaning “winter worm, summer grass”, is a literal translation of the original Tibetan name, yartsa gunbu. There are many colloquial names for this fungus, perhaps due to its historical significance in traditional Chinese medicine.
Description and Significance
O. sinensis is endemic to alpine regions and is found primarily on the Tibetan Plateau. The fungus parasitizes and mummifies the larva of several ghost moth species to form a fungus-caterpillar complex. This complex has been widely used in traditional Chinese medicine to treat a wide variety of ailments including asthma and respiratory inflammation [17]. The increase in commercial demand has led to excessive harvest and an extreme decline in the natural population; O. sinensis is currently listed as an endangered species in China.
Genome Structure
O. sinensis has a haploid genome that has not been sequenced entirely. Nevertheless, several important genes and sequences have been identified by recent genetic analysis. A shotgun genomic library was constructed from O. sinensis samples to determine part of its genome, to identify potentially useful markers, and to establish sequences that are specific to the species [5]. Although the genome is not yet complete, analysis of these markers and species-specific sequences can give insight into the evolutionary history of O. sinensis.
Gene Flow
Analysis of mating-type genes (MAT1) gives insight to the geographical gene flow of O. sinensis [7]. MAT1 has two alleles, or idiomorphs (MAT1-1, MAT1-2) that control sexual development of the fungus. These genes can also be found in many other fungal species, where they evolve rapidly; they tend to vary less within species and more between species. However, these genes vary greatly within the species for O. sinensis; this suggests an abnormally high degree of intraspecific genetic diversity, likely due to its unique geophysical environment and wide host range. The diversity of MAT1 genes were examined in isolates from different geographical locations [7]. There is considerably more diversity in isolates from the Southern part of the Tibetan Plateau than in Northern isolates; this suggests that O. sinensis was spread from the Southern locations to other locations.
Life Cycle
The life cycle of O. sinensis can be divided into the teleomorphic and anamorphic stages. The anamorphic stage results from the teleomorphic stage; molecular studies of microcycle conidation from ascopores have determined Hirsutella sinensis as the species for the asexual stage [16]. The fungus must infect a host insect in order to enter the teleomorphic, or sexual stage. The host insect species is a subterranean, root-boring insect that lives above altitudes of 3000m on the Tibetan Plateau. Ghost moth larvae of the genus Hepialus seem to be the most common hosts, although other potential hosts have been identified [3].
Host Infection
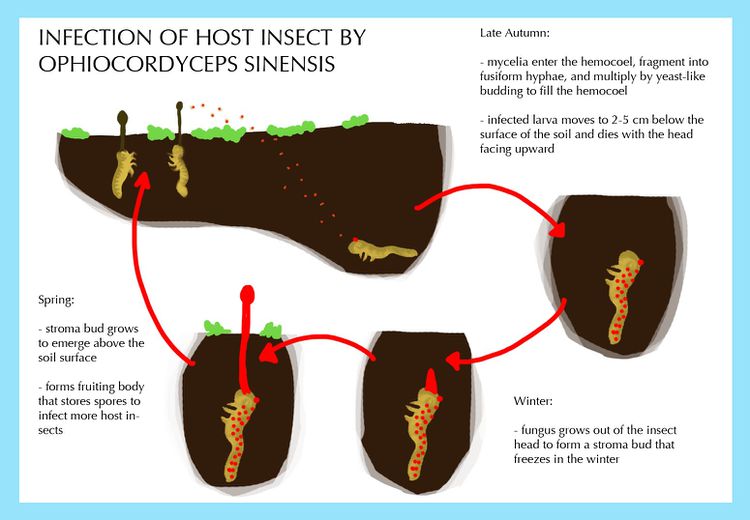
The fungus infects the underground larva in late autumn. Infection rates are highest in the 4th to 5th instar larvae that are shedding old cuticles and forming new ones. Less advanced larvae are less likely to be infected because they exhibit limited movement and food uptake, whereas larvae that have passed the 5th instar stage have increased resistance to fight the fungal infection. This stage in the larval life cycle overlaps temporally with the release of spores by O. sinensis [4]. The mycelia enter the hemocoel of the larva, fragment into fusiform hyphae, and multiply by yeast-type budding to fill the hemocoel [15]. The infected larva moves to 2-5 cm below the surface of the soil and dies with the head facing upward. The fungus grows out of the head of the insect host to form a stroma bud that freezes in the winter [8]. In the spring, the stroma bud grows to emerge above the soil surface and form the stalked fruiting body.
Other factors influence the infection rate of O. sinensis. The presence of other specific fungal species shortens the time required to mummify the host larva from 50 days to 3-5 days. This increases the infection rate from 3% to 60%. Infection is facilitated by loose soil and precipitation that allow the movement of ascospores deeper into the soil to be more accessible to the larva. Infection rates increase when large differences between day and night temperature increase the vertical movement of the larva [4].
Ecology
The Tibetan Plateau is a fragile ecosystem that exhibits high faunal and floral diversity as well as high endemism. It is the source of many important rivers in Asia, and its ecological fate affects many other environments. O. sinensis is found primarily on the Tibetan Plateau, but has also been documented in other Himalayan countries such as Nepal, Bhutan, and India [2]. The spread of O. sinensis depends on the shooting of ascospores from the fruiting bodies; this is limited to defined areas on the mountains, and the gene flow between various different populations is expected to be low. The need for O. sinensis to parasitize underground larvae, as well as its psychrophilic nature also limit the spread of the species to other environments.
O. sinensis is part of a tripartite relationship between the fungal species, the host insect it infects, and the plants that the insect consumes. This relationship seems to maintain itself in a relatively stable system. However, overharvesting the valuable fungus-caterpillar complex leads to a decrease in the fungal spores that are released, and an increase in the host insect population. Ghost moths in particular have been known for their ability to destroy plant populations in some ecosystems [13]. It is largely unknown how the increase in the ghost moth population, and the following increase in herbivory could alter the plant community in the Tibetan Plateau.
Economy
O. sinensis is one of the most valuable medicinal fungi in the world; the price has been rising in recent years, and currently it is worth more than its weight in gold [6]. Sales of naturally occurring O. sinensis specimen represent an important part of the GDP of local governments. 80% of families in major production areas collect caterpillar fungi, and this business comprises 50-80% of their total income (Ma, 2010). The high value of the caterpillar fungus has led to inter-village conflicts over the access to its grassland habitats. In some cases, these conflicts have led to deaths, as was the case in November 2011, when a court in Nepal convicted 19 villagers over the murder of a group of farmers during a fight over the caterpillar fungus [11].
Medicine
Melanin has been hypothesized to protect fungal species against environmental stress such as UV radiation and temperature by functioning as an extracellular redox buffer to neutralize oxidants generated by environmental stress. Specifically, melanin isolated from natural O. sinensis specimen exhibit an enhanced ability to chelate Fe2+ ions [1]. This confers a survival advantage to the fungus by helping to keep toxic ions out and concentrating essential ions near the cells.
Cancer
Secondary bioactive metabolites have also been isolated from fungal species other than O. sinensis that live on the fungus-caterpillar complex. Various epipolythiodioxopiperazines (ETPs) have been determined to inhibit proliferation and induce apoptosis of cancerous cells in immunodeficient mice [9]. Other molecules such as diketopiperazines have been found to be cytotoxic against cancerous cells [10]. The presence of these metabolites seem to support the traditional medicinal qualities of the fungus-caterpillar complex; however, more research is needed to conclusively determine the medicinal effects of naturally occurring O. sinensis on the human body.
References
Critical References
(1) Dong CH and Yao YJ. "Isolation, characterization of melanin derived from Ophiocordyceps sinensis, an entomogenous fungus endemic to the Tibetan Plateau." J Biosci Bioeng, 2012, DOI: 10.1016/j.jbiosc.2011.12.001
(2) Shresha B, Zhang WM, Zhang YJ, and Liu XZ. "What is the Chinese caterpillar fungus Ophicordyceps sinensis (Ophiocordycipitaceae)?" Mycology, 2010, DOI: 10.1080/21501203.2010.536791
(3) Wang XL and Yao YJ. "Host insect species of Ophiocordyceps sinensis: a review." ZooKeys, 2011, DOI: 10.3897/zookeys.127.802
(4) Yang YX, Yang DR, Shen FR, and Dong DZ. "Studies on Hepialid larvae for being infected by Chinese "insect herb" fungus (Cordyceps sinensis)" Zool Res, 1989, 10(3):227-231 [1]
(5) Zhang YJ, Bai FB, Zhang S, and Liu XZ. "Determining novel molecular markers in the Chinese caterpilar fungus Ophiocordyceps sinensis by screening a shotgun genomic library." Appl Microbiol Biotechnol, 2012a, DOI 10.1007/s00253-012-4028-x
(6) Zhang YJ, Li EW, Want CS, Li YL, and Liu XZ. "Ophiocordyceps sinensis, the flagship fungus of China: terminology, life strategy and ecology." Mycology, 2012b, DOI 10.1080/21501203.2011.654354
(7) Zhang YJ, Xu LL, Zhang S, Liu XZ, An ZQ, Wang M, and Guo YL. "Genetic diversity of Ophiocordyceps sinensis, a medicinal fungus endemic to the Tibetan Plateau: Implications for its evolution and conservation" BMC Evol Biol, 2009, 9:290. DOI 10.1186/1471-2148-9-290
Other References
(8) Buenz EJ, Banter BA, Osmundson TW, and Motley TJ. "The traditional Chinese medicine Cordyceps sinensis and its effects on apoptotic homeostasis." J Ethnopharmacol, 2005, 96(1-2):19-29
(9) Chen Y, Guo H, Du Z, Liu XZ, Che Y, and Ye X. "Ecology-based screen identifies new metabolites from a Cordyceps-colonizing fungus as cancer cell proliferation inhibitors and apoptosis inducers." Cell Proliferant, 2009, 42(6):838-847
(10) Guo HS, Hu HJ, Liu SC, Liu XZ, Zhou YG, and Che YS. "Bioactive p-terphenyl derivatives from a Cordyceps-colonizing isolate of Gliocladium sp." J Nat Prod, 2007, 70:1519-1521
(11) “ ‘Himalayan viagra’: six men get life for Nepal murders.” BBC, 2011 Nov 15.
(12) Liu ZY, Liang ZQ, and Xin ZH. "Microscopic re-observation of Cordyceps sinensis and study of its ascosporal development." Guizhou Sci, 2003, 21:51-57, 68
(13) Stock SP, Strong D, and Garnder SL. "Identification of Heterorhabditis from California with a new species isolated from the larvae of the ghost moth Hepialis californicus from the Bodega Bay Natural Reserve." Fundam Appl Nematol, 1996, 19(6):585-592
(14) Sung GH, Hywel-Jones NL, Sung JM, Luangsa-ard JJ, Shrestha B, and Spatafora JW. "Phylogenetic classificaiton of Cordyceps and the clavicipitaceous fungi." Stud Mycol, 2007, 57(1):5-59
(15) Zeng W, Yin DH, Li QS, and Li L. "The growth of Cordyceps sinensis (Berk.) Sacc. in the infection and parasitic phases." Mycosystema, 2006, 25(4):646-650
(16) Zhang WM, Li TH, Chen YQ, Qu LH, Zhong H, and Xu XP. "Molecular study on anamorph of Cordyceps sinensis from Tibet." Microbiology, 2002, 29:54-57
(17) Zhu JS, Halpern GM, and Jones K. "The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: part I." J Altern Complement Med, 1998, 289-303