Shock chlorination: Difference between revisions
| Line 30: | Line 30: | ||
==Success rates== | ==Success rates== | ||
In 1998, using Raman and ultraviolet spectroscopic radiation, scientists proved that the bactericidal activity of chlorine treatment was quantitatively linked to the concentration of the hypochlorous acid in the chemical solution. Further, the pH dependence discovered indicates an equilibrium amongst the chlorine, hypochlorous, and hypochlorite<sup>11</sup>. | In 1998, using Raman and ultraviolet spectroscopic radiation, scientists proved that the bactericidal activity of chlorine treatment was quantitatively linked to the concentration of the hypochlorous acid in the chemical solution. Further, the pH dependence discovered indicates an equilibrium amongst the chlorine, hypochlorous, and hypochlorite<sup>11</sup>. | ||
The success of shock chlorination, however, is not that simple. Due to the process of horizontal gene transfer (in which bacteria can pick up DNA from other species), some species of bacteria previous susceptible to chlorine are evolving to be resistant. | |||
==Related and Alternative methods== | ==Related and Alternative methods== | ||
Revision as of 02:10, 2 December 2013
Introduction
From swimming pools to wells, chlorine is a common chemical used to disinfect water sources. About a century ago, the process of chlorination was introduced in order to prevent the spread of diseases such as typhoid or cholera. Over time, the process has modified, with the EPA (Environmental Protection Agency) beginning to implement the use of chloramine, a closely related compound, instead1.
Due to safety concerns, hypochlorite (bleach) is the most commonly used compound to conduct shock chlorination2. Hypochlorite is used in one of three forms: commercial bleach (approx. 3.5-5% concentration), calcium hypochlorite (Ca(OCl)3; 65-70% concentrated), or sodium hypochlorite (NaOCl; about 12% concentration)2. These forms of hypochlorite are not as pure as chlorine gas, and will degrade in strength when in storage. However, like chlorine gas, they also react with water to form a disinfectant, hypochlorous acid (HOCl).
Microbial agents
Frequently, microbial factors infiltrate water sources through fecal matter. Many types of bacterial pathogens can initiate waterborne illnesses, including enteric bacteria, protozoa, or viruses4.
Due to the variety of species that can inhabit water sources, it comes as no surprise that chlorine has varying affects on the types of microbes. Though time and concentration can eliminate virtually every species, some species remain resistant to the process. Species such as Vibrio cholera have been tested to last approximately 30 days in drinking water sources, while toxigenic E.coli can last 90 days5. Survival techniques include cysts, spores, absorption, and intracellular survival and growth. Other species will remain viable, but no longer culturable.
Helicobacter pylori
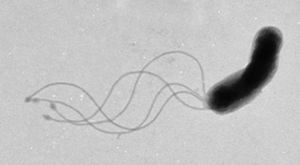
Helicobacter pylori is known to cause gastritis and peptic ulcers.
Studies done in Peru6 and Japan7 have shown the presence of the bacteria in public water sources, proving its possibility as a waterborne microbe.
Cryptosporidium
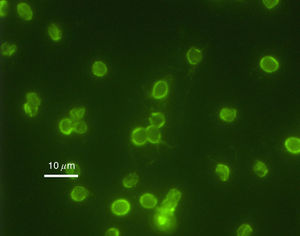
Cryptosporidium parvum is a type of parasite capable of causing gastrointestinal illness. Unlike Helicobacter pylori, however, Cryptosporidium has been proven to be unresponsive to most chlorination8.
In 1993, Milwaukee (Wisconsin) experienced one of the worst waterborne outbreaks after one of the city's water plants failed to remove Cryptosporidium oocysts from the Lake Michigan water collected for public use. Over 403,000 residents and visitors were affected, experiencing the watery diarrhea, nausea, fever, vomiting, and abdominal cramps associated with infection. Approximately 54 deaths occurred, although there is some debate regarding other preexisting conditions that affected patient survival9.
In October 2013, a resurgence of the Cryptosporidium protozoan appeared in Milwaukee, this time in local swimming pools10.
Methods
Commercial
Domestic
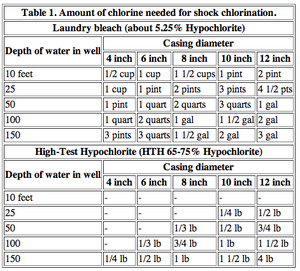
Success rates
In 1998, using Raman and ultraviolet spectroscopic radiation, scientists proved that the bactericidal activity of chlorine treatment was quantitatively linked to the concentration of the hypochlorous acid in the chemical solution. Further, the pH dependence discovered indicates an equilibrium amongst the chlorine, hypochlorous, and hypochlorite11.
The success of shock chlorination, however, is not that simple. Due to the process of horizontal gene transfer (in which bacteria can pick up DNA from other species), some species of bacteria previous susceptible to chlorine are evolving to be resistant.
Related and Alternative methods
Scientists are not content with shock chlorination. As technology advances, methods to improve both testing and disinfection are created.
Quality Monitoring
DNA microarray can be used to monitor water quality more precisely12.
Chlorine Replacements
Various groups complain about the toxic residues created by chlorination. However, various alternatives that have been created appear to have faults of their own, producing the carcinogenic compounds similar to the ones scientists sought to remove.
References
1 "Chlorine Substitutes In Water May Have Risks". 7 January 2011. NPR.
Edited by Erika Jensen, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2013, Kenyon College.
