Overview of Rhizopus oryzae
1. Classification
a. Higher order taxa
Domain: Eukarya, Kingdom: Fungi, Phylum: Zygomycota, Class: Zygomycetes, Order: Mucorales, Family: Mucoraceae, Genus: Rhizopus, and Species: oryzae.
2. Description and significance
Rhizopus oryzae is a species of filamentous fungi within the group Mucormycetes, a body of organisms largely found in decaying organic matter and responsible for causing infections in immunocompromised individuals [1]. It is the principal causative agent of the third most common invasive fungal infection in humans, after aspergillosis and candidiasis, known as mucormycosis. One of the most common comorbidities of this infection is diabetes, which continues to affect the health and well-being of millions both nationally and globally [1]. In addition to mucormycosis, R. oryzae causes approximately 90% of the infectious complications of cancer patients, in particular those with hematological malignancies [2]. Oftentimes, these infectious complications are difficult to identify and diagnose early on due to the initial occurrence of asymptomatic phases, and as a result treatment is delayed [2]. Thus, such a microbe is highly relevant to the safety and care plans of modern human populations. By understanding the genetic structure and biological processes associated with the microbe’s reproductive gene loci, knowledge can be obtained regarding disease inhibition and then applied to medicinal developments to allow for early detection and treatment [2].
3. Genome structure
The genome of R. oryzae strain 99-880, which measures 45.3 Mb in length, contains numerous transposable elements (TEs) and coding sequences [3]. These TEs and coding sequences comprise 20% and 39% of the genome, respectively. The TEs of R. oryzae can be categorized into two classes: Class I and Class II. Class I TEs contain retrotransposons, while Class II TEs include DNA TEs [3]. In addition to TEs and coding sequences, the genome of R. oryzae contains expanded gene families involved in several processes such as cell wall synthesis, protein hydrolysis, and cell signaling [3]. The presence of the protease gene family in particular indicates a greater ability of R. oryzae to degrade organic matter. Additionally, the increase of the CHS and CDA gene families to 23 and 34 genes, respectively, accounts for the increase in chitin and chitosan within the microbe’s cell wall [3]. Other gene families found in R. oryzae transcribe proteins such as GTPases, GTPase regulators, chitin synthases (CHS), chitin deacetylases (CDA), secreted aspartic proteases, and subtilases [3]. Compared to other fungal microorganisms, R. oryzae possesses a highly repetitive genetic structure, as it contains ancestral genes similar to those contained in metazoan genomes rather than dikaryotic fungi genomes [3]. Such findings suggest that the genomic structure of R. oryzae observed today occurred as a result of whole genome duplication and subsequent sequence deletions [3].
4. Cell structure
R. oryzae grows as a result of mycelium accumulation [4]. Such accumulations are composed of tubular filaments known as hyphae. Mycelial cell walls, located in the body of the microbe, are reinforced by polysaccharides such as chitin [4]. Chitin reinforces the hyphae, creates a stronger cell wall, and thus allows for the cell to withstand greater pressures. While chitin provides a source of external, structural support to this microbe’s cells, lipids provide a source of internal, metabolic support as they can act as cellular storage sites [4].
5. Metabolic Processes
Energy Catalysis
Microbes belonging to the Rhizopus genus are optimal organisms for fumaric acid production [5]. R. oryzae, in particular, produces and accumulates large quantities of fumaric acid under aerobic conditions via the tricarboxylic acid cycle. Such cycling occurs in the cytosol through use of the C3 and C1 mechanism, which works in conjunction with the carbon dioxide fixation pathway, to allow for the biosynthesis of fumaric acid [5]. The process begins when pyruvate is catalyzed by pyruvate carboxylase into oxaloacetate. The catalysis reaction is continued by malate dehydrogenase, and later by fumarase, to break down L-malic acid and ultimately produce fumaric acid [5]. Acidic stress on cells can occur during acid production, which decreases the pH in the fermentation broth [6]. A pH of 4.0 has been reported to be favorable for the synthesis of fumaric acid by R. oryzae. At a pH of 4.0, R. oryzae exhibited rapid glucose consumption and a higher output of both fumaric acid and biomass than at a pH of 3.0 [6]. Glucose was also completely consumed by the end of the process and twice as much fumaric acid was produced than at a pH of 3.0. On the contrary at a pH of 3.0, acid production significantly decreased and some glucose remained in the broth [6]. The decreased acid levels at pH 3.0 demonstrate that low pH inhibits fumaric acid production by R. oryzae. Acid production may be inhibited due to limited availability of carbon sources that are alternatively used for cell survival processes in low pH conditions [6].
Reproduction
Current knowledge about the reproductive process of R. oryzae is primarily derived from observing and comparing different species under the group Rhizopus. R. oryzae has a heterothallic mating arrangement, meaning that offspring may be produced following reproduction between two compatible spore types [2]. The method by which R. oryzae reproduction occurs is dependent upon the available media type. In high nutrient environments, this microbe can reproduce asexually through mitosis, the most common reproductive method used by the species [2]. In minimal nutrient environments, diploid cells produce spores through meiosis. These spores can grow as azygospores, which are produced through asexual reproduction, or zygospores, which are produced through haploid cell fusion [2]. Mating can occur between different species as well. For instance, R. oryzae and R. delemar can act as mating partners as their zygospores are physically and molecularly similar. [2] Both microbes contain central vacuoles and produce round, reddish brown zygospores. In order to form spores, both require little to no light and an incubation period of about two to three weeks [2]. The crossing of R. oryzae and R. delemar results in offspring that cannot create zygospores due to the presence of a conserved sex locus, contained in the genome strain RA99-880 [2].
6. Ecology
The microbe R. oryzae can be classified within the phylum Zygomycota [7]. About 1050 species of different fungi have been recognized as part of this phylum, and can be further subdivided into two categories: Zygomycetes and Trichomycetes [8]. The class Zygomycetes, which includes R. orzyae, contains many different fungal groups such as plant and animal (non-human) pathogens and saprobes [7]. These fungal groups can reproduce through various processes including, but not limited to, asexual reproduction through sporangiospores, sexual reproduction through zygospores, and the formation of hyphae which do not separate through a septum [7]. The multiple reproduction methods that R. oryzae is able to utilize allows it to be found throughout nature; it has previously been isolated within soil, decaying vegetables and citrus fruits, and seeds of tropical and subtropical regions [8]. Its ability to quickly infect substances high in sugar content also allows it to be identified as a primary or secondary colonizer [1].
7. Pathology
How to Detect the Organism
As of today, it is not easy to detect the fungus. There are no known serological tests that effectively detect R. oryzae [9]. Typically, a biopsy is performed after a patient comes in with symptoms. In the event of a possible pulmonary invasion, biopsies are difficult to perform because they could result in internal bleeding and pneumothorax [9]. As a result, a bronchoalveolar lavage—in which a bronchoscope is inserted through the mouth or nose and it goes into the lungs—is performed. Once the bronchoscope reaches its destination, it will release a solution to wash the area to collect and test for the fluid contents [9]. The most effective way to test fluid for Mucormycetes is through the use of a real time quantitative polymerase chain reaction/High Resolution Melting Analysis (RQ - PCR/ HRMA) method. During this process, DNA is amplified and bound to a fluorescence marker [9]. The change in fluorescence is then observed as the product is melted [9]. From this data, a curve can be created, and the pattern produced can determine the severity of an individual’s infection with mucormycosis [10]. Such a curve also allows doctors to detect the microbe with 99% accuracy [10].
Pathogenic Diseases
Mucormycosis, a life-threatening infection that occurs in immunocompromised individuals, is most commonly caused by R. oryzae [11, 12, 13]. The infection occurs when the fungus invades the blood or lymph vessels in a process known as angioinvasion [11]. This results in thrombosis and subsequent necrosis of the infected tissues. The ability of the infection to spread and cause extensive tissue damage even before detection and diagnosis contributes to its high mortality rate (approximately 50%) [11]. Risk factors associated with mucormycosis include iron overload, prolonged neutropenia and use of corticosteroids, human immunodeficiency virus infection, malnutrition, burns, wounds, myelodysplastic syndromes, aplastic anaemia, and deferoxamine use [14].
Invasive mucormycosis has five major forms: rhino-orbitocerebral (ROCM), pulmonary, cutaneous, gastrointestinal, and disseminated [14]. Rhino-orbitocerebral mucormycosis is the most common form, particularly in patients with uncontrolled diabetes mellitus [12, 14]. It has a mortality rate of approximately 85% and includes symptoms such as nasal congestion, headache, maxillary pain, odontalgia, blurred vision, double vision, proptosis and blindness [14, 15]. Pulmonary mucormycosis is most common in neutropenic patients or cancer patients [14]. Symptoms include prolonged fever that is unresponsive to antibiotics, pleuritic chest pain, coughing, and deteriorating dyspnea. Cutaneous mucormycosis occurs after the fungus enters the body through skin breaks [14]. Symptoms include the presence of necrotic eschars, which should immediately prompt a skin biopsy. Gastrointestinal mucormycosis occurs when fungal spores are ingested (via contaminated foods), and is more common among neonates [14]. Symptoms include necrotizing enterocolitis with distention, redness, and tenderness in the abdomen region. Disseminated mucormycosis occurs when infection from the initial site spreads to other parts of the body via the bloodstream [14].
In addition to causing mucormycosis, R. oryzae spores and germ tubes can adhere to and damage human umbilical vein endothelial cells (HUVECs) [11]. The fungus does not need to be viable to damage HUVECs. Therefore, the human body can still become infected after the R. oryzae cells die [11].
Possible Treatment Methods
Because R. oryzae is difficult to detect, early diagnosis and appropriate therapy are crucial to treating patients with mucormycosis [12]. One treatment method is the use of antifungal drugs to inhibit microbial growth and activity [12, 16]. When tested against clinical isolates of Mucorales, the most potent antifungal medication was Amphotericin B, followed by Posaconazole, Itraconazole, and Isavuconazole [12]. Amphotericin B is a broad-spectrum antifungal polyene [17]. Posaconazole can treat ROCM and is a potential cure for adult diabetic patients with this infection [16].
A potential therapeutic strategy for mucormycosis is iron starvation [13]. To induce physiological stress, iron starvation activates the enzyme metacaspase, decreases cellular mitochondrial membrane potential, and inhibits oxidative phosphorylation of R. oryzae FTR1::RNAi mutants, ultimately causing apoptosis [13].
7. Key microorganisms
Include this section if your Wiki page focuses on a microbial process, rather than a specific taxon/group of organisms
8. Current Research
"R. oryzae" can cause postharvest disease in certain fruits such as apples and bananas [18]. This disease originates from soft rot, a fungal infection that occurs when products are stored at nonoptimal temperatures or extended periods of time at cold temperatures. When such fruits rot, white and cottony "R. oryzae" colonies will form on their surfaces [18].
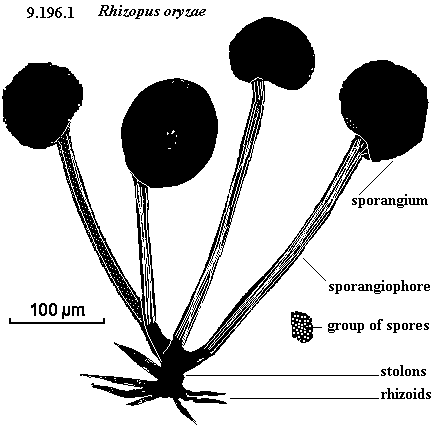 Visible structure of "R. oryzae" – sporangium is typically visible on decaying food as fuzz.
http://www.uq.edu.au/_School_Science_Lessons/9.196.1.GIF
Visible structure of "R. oryzae" – sporangium is typically visible on decaying food as fuzz.
http://www.uq.edu.au/_School_Science_Lessons/9.196.1.GIF
Although "R. oryzae" is a pathogenic microbe, it can be useful in food production [19]. Commercial "R. oryzae" cultures can be cooked and used as dry starters to inoculate different fruits and grains. As a fermentative agent, this microbe can enhance sweetness and acidity while producing a white, protective covering on food surfaces [19]. Although such food covers are edible, further research is needed to determine whether these products are safe to incorporate into the food industry completely.
The new "R. oryzae" strain FSIS4 also produces an 𝛼-amylase enzyme that increases the volume and height-to-width ratio of bread [20]. Once purified via a three-phase partitioning technique, the enzyme is added to wheat flour at a concentration of 1.936 U per kg of flour. The increase in volume and height-to-width ratio may occur due to a reduced viscosity of the dough during starch gelatinization [20]. An aerated bread structure also results from the presence of 𝛼-amylase produced by the microbe. Bread utilizing commercial 𝛼-amylase creates a similar aerated structure, however with larger holes [20]. Upon further research, the effects of "R. oryzae" FSIS4 𝛼-amylase may prove useful in other food products as well.
9. References
1. Bala, K., Chander, J., Handa, U., Punia, R. S., and Attri, A. K. 2015. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Medical Mycology 53(3): 248–257.
2. Gryganskyi, A. P., Lee, S. C., Litvintseva, A. P., Smith, M. E., Bonito, G., Porter, T. M., Anishchenko, I. M., Heitman, J., and Vilgalys, R. 2010. Structure, function, and phylogeny of the mating locus in the Rhizopus oryzae complex. PLoS One 5(12):15-273.
3. Ma, L., Ibrahim, A. S., Skory, C., Grabherr, M. G., Burger, G., Butler, M., Elias, M., Idnurm, A., Lang, B. F., Sone, T., et al. 2009. Genomic Analysis of the Basal Lineage Fungus Rhizopus oryzae Reveals a Whole-Genome Duplication. PLOS Genetics 5(7):e1000549.
4. Mélida, H., Sain, D., Stajich, J. E., and Bulone, V. 2015. Deciphering the uniqueness of Mucoromycotina cell walls by combining biochemical and phylogenomic approaches. Environmental Microbiology 17:1649-1662.
5. Yin, X., Li, J., Shin, H., Du, G., Liu, L., and Chen, J. 2015. Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms: Advances and prospects. Biotechnology Advances 33(6):830-841.
6. Liu, Y., Xu, Q., Lv, C., Yan, C., Li, S., Jiang, L., Huang, H., and Ouyang, P. 2015. Study of metabolic profile of Rhizopus oryzae to enhance fumaric acid production under low pH condition. Applied Biochemistry and Biotechnology 177(7):1508-1519.
7. Londoño-Hernández, L., Ramírez-Toro, C., Ruiz, H. A., Ascacio-Valdés, J. A., Aguilar-Gonzalez, M. A., Rodríguez-Herrera, R., and Aguilar C. N. 2017. Rhizopus oryzae–Ancient microbial resource with importance in modern food industry. International Journal of Food Microbiology 257:110-127.
8. Hunter, W. E., Duniway, J. M., and Butler, E. E. 1977. Influence of nutrition, temperature, moisture, and gas composition on parasitism of Rhizopus oryzae by Syncephalis californica. Phytopathology 67:664-69.
9. Lengerova, M., Racil, Z., Hrncirova, K., Kocmanova, I., Volfova, P., Ricna, D., Bejdak, P., Moulis, M., Pavlovsky, Z., Weinbergerova, B., et al. 2014. Rapid detection and identification of mucormycetes in bronchoalveolar lavage samples from immunocompromised patients with pulmonary infiltrates by use of high-resolution melt analysis. Journal of Clinical Microbiology 52(8):2824-2828.
10. Dolatabadi, S., Kolecka, A., Versteeg, M., de Hoog, S. G., and Boekhout, T. 2015. Differentiation of clinically relevant Mucorales Rhizopus microsporus and R. arrhizus by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). Journal of Medical Microbiology 64:694-701.
11. Ibrahim, A. S., Spellberg, B., Avanessian, V., Fu, Y., and Edwards, J. E., Jr. 2005. Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infection and Immunity 73(2):778-783.
12. Chowdhary, A., Kathuria, S., Singh, P. K., Sharma, B., Dolatabadi, S., Hagen, F. and Meis, J. F. 2014. Molecular characterization and in vitro antifungal susceptibility of 80 clinical isolates of mucormycetes in Delhi, India. Mycoses 57(3): 97–107.
13. Shirazi, F., Kontoyiannis, D. P., and Ibrahim, A. S. 2015. Iron starvation induces apoptosis in Rhizopus oryzae in vitro. Virulence 6(2):121–126.
14. Mantadakis, E., and Samonis, G. 2009. Clinical presentation of zygomycosis. Clinical Microbiology and Infection 15(5):15-20.
15. Roden, M. M., Zaoutis, T. E., Buchanan, W. L., Knudsen, T. A., Sarkisova, T. A., Schaufele, R. L., Sein, M., Sein, T., Chiou, C. C., Chu, J. H., et al. 2005. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clinical Infectious Diseases 41(5):634-653.
16. Manesh, A., John, A. O., Mathew, B., Varghese, L., Rupa, V., Zachariah, A., and Varghese, G. M. 2016. Posaconazole: an emerging therapeutic option for invasive rhino-orbito-cerebral mucormycosis. Mycoses 59(12):765–772.
17. Tang, X., Zhu, H., Sun, L., Hou, W., Cai, S., Zhang, R., and Liu, F. 2014. Enhanced antifungal effects of amphotericin B-TPGS-b-(PCL-ran-PGA) nanoparticles in vitro and in vivo. International Journal of Nanomedicine 9:5403–5413.
18. Kwon, J., Ryu, J., Chi, T. T., Shen, S., and Choi, O. 2012. Soft rot of Rhizopus oryzae as a postharvest pathogen of banana fruit in Korea. Mycobiology 40(3):214-216.
19. Cantabrana, I., Perise, R., and Hernández, I. 2015. Uses of Rhizopus oryzae in the kitchen. International Journal of Gastronomy and Food Science 2(2):103–111.
20. Ait Kaki El-Hadef El-Okki, A., Gagaoua, M., Bourekoua, H., Hafid, K., Bennamoun, L., Djekrif-Dakhmouche, S., El-Hadef El-Okki, M. E., and Meraihi, Z. 2017. Improving bread quality with the application of a newly purified thermostable α-amylase from Rhizopus oryzae FSIS4. Foods 6(1).
