Photorhabdus luminescens Toxins as Therapeutic Agents
Introduction

By [Oliver Kendall]
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC. Every image requires a link to the source.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
P. luminesces is the only terrestrial bacteria to engage in photodynamic reactions. This bacteria is a grahm negative nematode-symbiote that primarily infects insects[1]. P. luminesces lives in Heterorhabditidiae intestine, and is regurgitated into the haemocoel of the insect host. The bacteria then switches on its pathogenic genes, excreting toxins that kill the host. Once the host is dead, P. luminesces continues to produce antibacterial agents to maximize nutrient uptake for the nematode to then unsure further propagation. P. luminesces has three different toxins that it excretes when in its pathogenic state. The toxins are: Toxin Complexes (Tcs)[2], Photorhabdus insect related (Pir) proteins, and the “makes caterpillars floppy” (Mcf) toxins[1][3]. These toxins use different methods to infect the insect, and as a result are activated by different factors. Tcs primarily use a novel ABC-like transporter to inject toxins into the midgut cells of the insect[2]. Pir proteins also inject toxins into the insect, but act as neurotoxins. Mcf toxins act to lyse the midgut and internal organs of the insect while also removing body turgor to make the caterpillar “floppy”. Regulation of the expression of these toxins is important for both P. luminesces and its nematode host. Incorrect activation of the genes that encode for any one of these toxins would kill the nematode, and eventually kill the bacteria due to its need for the symbiotic relationship.
Toxins produced by P. luminesces are not the only important part of this bacteria. In its life cycle, once the insect host has been killed, P. luminesces expresses antibacterial agents to maximize the nutrients for its nematode-symbiote. These antibacterial agents have not been widely studied until recently, and could provide the answer for previously untreatable infections or diseases.
Sample citations:
[4]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
To repeat the citation for other statements, the reference needs to have a names: "[5]"
Genomic Regulation of Pathogenicity
Pathogenicity of P. luminesces is essential to the survival of both P. luminesces and the nematode. This pathogenicity must be closely regulated so that the nematode is not killed, preventing propagation of the bacteria. The nematode regurgitates P. luminesces into the haemocoel of the insect, where the bacteria’s pathogenic genes are activated[2]. P. luminesces nullifies the host cellular immunity, while avoiding antimicrobial peptides (AMPs)[5]. Once the host is dead, P. luminesces helps in the digestion of the host, while producing antibacterial agents to prevent growth of other bacteria. Two genotypic forms of P. luminesces have been found, but only type 1 supports the symbiotic relationship with the nematode[1][5][2].
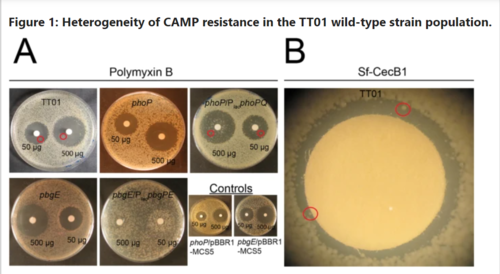
Switching from symbiotic to pathogenic states with P. luminesces is indicated by expression of fimbriae on the cell. In the on state, the cell is covered with the fimbriae which is associated with the symbiotic state of P. luminesces. In the off state, the cell loses its fimbriae which only occurs in the pathogenic state[5]. Loss of the fimbriae is not the only phenotypic change that occurs in the transition to the pathogenic state. In terms of the pathogenic genes, host insects express cationic antimicrobial peptides (CAMPs). These cationic peptides interact with the anionic surface of bacterial cells, by binding to the acidic A site on LPS[5].
In order to infect the host insect, P. luminesces generates a subpopulation with CAMP resistance to ensure septicemia[5]. Wild type proteins were found to express genes related to CAMP resistance using agar disk diffusion assays. P. luminesces that specifically express phoP and pdgPE mutations demonstrate CAMP resistance[5]. Wild type P. luminesces have been shown to express CAMP resistance mutants in the absence of CAMPs at low levels (0.5% of population). Expression of these mutations is only upregulated to 1% of the population in the presence of CAMPs in vitro, which means that CAMP resistance is not entirely dependent on mutations[5].
P. luminesces CAMP resistance is regulated by the PhoP regulon. Expression of this regulon can be observed in vitro by adding low levels of Mg+ to imitate the presence of CAMPs[5]. The PhoP regulon encodes genes for CAMP resistance, virulence, and bacterial antagonism, meaning that to switch to the pathogenic phase all P. luminesces needs to phosphorylate the PhoP protein, bringing it to the active state[5].
CAMP resistance in P. luminesces is closely correlated with insect death. Low levels of expression of PhoP regulated genes ensures that injection into the haemocoel of the insect does not kill all P. luminesces. Large numbers of P. luminesces are killed by CAMPs, which allows for rapid growth of CAMP resistant variants. Septicemia in the insect is observed once CAMP resistant variants begin to outcompete CAMP susceptible variants[5].
CAMP resistance develops randomly in the absence of CAMPs, but has been shown to be heritable. In the presence of CAMPs, this random development causes CAMP resistant variants to outcompete the susceptible variants. Since virulence genes are regulated by the same PhoP protein activation of CAMP resistance also activates virulence. Septicemia then occurs as a result of CAMP resistant variants outcompeting other variants[5].
Classification of Toxins
P. luminesces forms pathogenic and symbiotic relationships, and must be able to switch these functions on and off to ensure its survival. Within the nematode, P. luminesces lives within the intestine[1][3]. It is then released into the bloodstream or haemocoel of the host, where it becomes pathogenic[2]. This bacterium has been found in two different phenotypic stages. In stage 1, P. luminesces lives within the nematode intestine, where it produces antibiotics, and a variety of other compounds for the nematode. Phase 2 variants are only found in vitro and do not produce most of these compounds, but still express pathogenic proteins. With these proteins, <P. luminesces has four genomic islands related to its pathogenicity. Three of these correspond with toxins that are injected into the host, and the fourth encodes the transport complex[1][3].
The P. luminesces toxins are: Toxin Complexes (Tcs)[2], Photorhabdus insect related (Pir) proteins, and the “makes caterpillars floppy” (Mcf) toxins[1]. P. luminesces Tcs are discussed in the next section. Pir toxins are binary proteins that are only functional when both A and B subunits are present. Located at plu4093-4092 (pirA) and plu4437-4436 (pirB) loci of P. luminescens TT01 genome, these genes encode proteins involved in injection of toxins into host cells. PirAB have been found to be structurally similar to juvenile hormone esterase-like (JHE-like) proteins. These proteins act as neurotoxins that promote the influx of Ca2+ and the release of neurotransmitters from presynaptic nerve terminals. Due to the similarity between these two proteins, it is likely that the PirAB toxins kill the insect in a similar way[1][3].
The final toxin that P. luminesces produces to kill host insects are the “make caterpillars floppy” toxin 1 (Mcf1)[1][3]. This toxin was discovered through gene transfer to E. coli. When expressed in the E. coli, the mcf1 gene caused the lysis of the midgut, making the caterpillar look floppy. Mcf1 toxins induce apoptosis by attacking the mitochondria. Mcf1 toxins are structurally similar to viral BH3 proteins that bind to the mitochondria to prevent the release of anti-apoptotic proteins. This was done through mutagenesis of the BH3 binding site on the mitochondria. This effectively prevented apoptosis with BH3 and mcf1 toxins. This is especially important because then Photorhabdus are then the first bacteria to synthesize pro-apoptotic proteins similar to viral BH3[3].
The toxins produced by P. luminesces have the potential to be antibacterial agents in terms of agriculture. Mcf1 being the exception to this because these toxins cause apoptosis in mammalian cells, so they would not be useful in agriculture. Important applications of the Tcs proteins involve the integration of the ABC Tc into plant genome provided the plant with immunity from insects. When integrated into the genome, these toxin-encoding genes can provide the plant with immunity from a variety of different pests. This is possible because the BC genes can cross-potentiate, and produce two variants of the A toxin when separated on the genome[3]. This allows for immunity against a wider variety of insects. This increase in toxicity comes from some organisms having immunity against a certain A toxin, but an absence of immunity against the other A toxin. This research has potential therapeutic implications as well. These Tc and Pir proteins have been shown to be antibacterial agents that can be integrated into the genome and expressed in multiple different organisms. Although using mechanisms like CRISPR-Cas9 to incorporate Tc and Pir genes in the human genome, the lack of specificity with these toxins prevent these from being viable therapeutic agents. The problem with this is that some of these proteins only release their toxins upon acidification, or alkylation, so the toxins would primarily be released in the human gut. This has the potential to kill the human gut microbe.
Tcs Toxin and Injection Mechanism
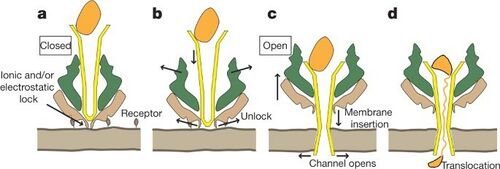
Upon entering the target insect, P. luminesces excretes multiple different toxins using unique tripartite ABC transport complexes (Tcs)[1][3][2]. This ABC transport complex is only functionally active when all three parts are present. These Tcs proteins facilitate the translocation of TcC into new host cell using TcA and TcB. TcC has been found to be the toxin that is injected into the new cell, resulting in actin clustering, phagocytosis, and cell death[1]. The structure of the Tcs protein was thought to be primarily beta barrel in structure, but recent studies have shown that the central part of the complex is made of 25 alpha helices. The upper part of the pore is made of five beta sheets, with the pore being 1.41MD and 25.5nm long. Results were obtained using Cryo-EM and show major structural differences between the pore and prepore states. In transitioning from the prepore to the pore state the central pore moves 115 Å towards the lower end of the complex, and the tube portion penetrates the membrane[2].
Insertion of the Tcs was monitored in vitro, showing little insertion indicating that insertion of the P. luminesces Tcs is dependent upon a the presence of a receptor on the host cell[2]. Transition from the prepore to pore state was monitored in vivo, proving this. Recent research has shown that in vitro in the absence of this receptor the transition can be induced by changes in pH. Researchers found that pH 4 and 11 induced conformational changes that represented the pore state as a result of protonation of residues like aspartate or glutamate, and the deprotonation of residues like tyrosine, or lysine. In vivo this is shown to also disrupt electrostatic and ionic interactions on the outer shell of the Protein Translocation Complex(PTC)[2]. This indicates that the toxins are most likely translocated in very acidic or basic sites. Researchers found that in nature, P. luminesces are released in the haemocoel, where they colonize midgut cells. The Protein Translocation Complex(PTC) binds as a preprotein to midgut cells after being released into the haemocoel. The electrostatic interactions that prevent pore formation are released upon acidification with the endosome, or alkylation in the midgut. This release causes the central pore to open, and penetrate the membrane. This pore opening and penetration of the membrane induces the translocation of TcC into the host cell.
Therapeutic Agent for Zoonotic Diseases
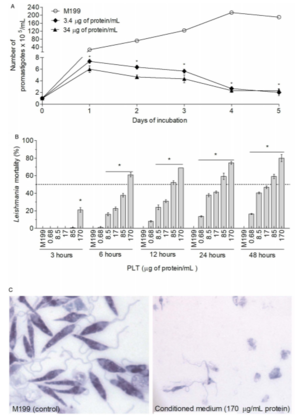
P. luminesces excretes toxins that can be used as therapeutic agents to combat previously resistant zoonotic diseases[6]. Zoonotic diseases are parasitic infections from close contact animals. Leishmaniasis is a zoonic parasitic disease caused by sand flies. This disease is also known as the black fever, and affects people all around the world[7]. Previously this disease’s treatment options are very expensive, and have minimal effect, as the pathogen has the possibility for resurgence. P. luminesces is a potential treatment option because of the variety of compounds excreted in its pathogenic state, and the efficacy of these toxins with preventing other pathogenic growth. P. luminesces has been shown to develop toxins that greatly inhibit parasite growth[6][7].
Photorhabdus-derived leishmanicidal toxin (PLT) were shown to kill leishmaniasis parasites with an Ic50 of 21.8µg *M-1. Treatment of proteinase k drastically inhibited PLT activity illustrating that PLT must be DNA or RNA based. To further support this, acidification of the culture drastically inhibited PLT activity, whereas at values above pH 8 PLT was able to kill the parasites. Using ultrafiltration, PLT was determined to have molecular weight smaller than 3 kDa[6]. PLT affects leishmaniasis by depolarizing the mitochondrial membrane[8]. This results in a decrease in APT production for the parasite because the depolarization of the membrane affects the cells ability to participate in the TCA cycle. Importantly this phenomenon is only observed in the leishmaniasis parasite as PLT has low cytotoxicity for human and mammalian cells.
Leishmania parasites have been shown to live in two different stages, as an amastigote, and as promastigote[7]. Due to the lack of current research on structural differences leishmania is referred to as promastigote when in the sand fly, and as an amastigote when in a mammal. PLT has been shown to be more selective for the amastigote, which means that it is a potential therapeutic agent for human infections[6].
Therapeutic Agent for Psoriasis
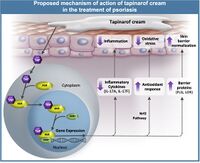
P. luminesces has also been shown as a potential treatment option for less severe diseases like psoriasis[4]. This disease results in skin cells multiplying 10 times faster than the normal growth rate. The growths can happen anywhere and result in bumpy red patches with white scales. This disease can lead into certain types of arthritis, but is now treatable using a compound derived from P. luminesces. One of the metabolites produced after the insect is killed by P. luminesces was isolated and determined to be 3,5-dihydroxy-4-isopropylstilbene[4]. This has been pharmaceutically developed and named Tapinarof. Tapinarof has been found to bind to AhR which downregulates the production of cytokines, regulation of the skin barrier protein expression, and antioxidant activity. In vivo Tapinarof directly inhibits IL17A, IL17F, IL19, IL22, IL23A, and IL1B cytokines by binding to AhR[4]. Tapinarof also induces the expression of keratinocytes, which helps to repair restore epidermal function. Psoriasis severity is determined by the presence of reactive oxidative species (ROS), and the absence of antioxidants[4]. Since Tapinarof is a stilbene molecule it readily picks up ROS forming superoxide anions and hydroxyl radicals. Presence of Tapinarof also activates the AhR-Nrf2 transcription factor pathway that produces antioxidants[4].
Conclusion
P. luminesces life is characterized by two different stages: symbiotic, and pathogenic. In the symbiotic stage, the bacteria produce three primary toxins[1][3][2]. These toxins only affect the infection and death of the insect host. By nature these compounds are not suitable for therapeutic agents. On the other hand, PhoP regulates a wide variety of genes that have yet to be fully characterized. P. luminesces produces compounds that act as antimicrobial agents once the insect host has been killed. Uncharacterized compounds that serve as antimicrobial agents in the P. luminesces infection of insects have the potential to be therapeutic agent for historically resistant pathogens.
An example of one of these previously uncharacterized compounds is PLT. This <3-kDa peptide has been shown to drastically inhibit infection of the amastigote variant of leishmania[7][6]. It is yet unclear if PLT is specific to leishmania infections, but the possibility for inhibition of other zoonotic infections calls for further research. In addition, compounds derived from P. luminesces like Tarpinof have been shown to drastically inhibit psoriasis. This compound that has been pharmaceutically developed has multiple different methods of reducing psoriasis symptoms[4]. This has major implications for medicine because the anti-inflammatory properties of tapinarof resulting from the downregulation of cytokine activity has potential other therapeutic uses. For example instead of a topical agent, if tapinarof could be made into an aerosol then it could be inhaled as a treatment option for cytokine induced inflammation like asthma.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Rodou A, Ankrah DO, Stathopoulos C. Toxins and secretion systems of Photorhabdus luminescens. Toxins (Basel). 2010;2(6):1250-1264.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 [https://www.nature.com/articles/nature11987 Gatsogiannis, C., Lang, A., Meusch, D. et al. A syringe-like injection mechanism in Photorhabdus luminescens toxins. Nature 495, 520–523 (2013).
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 [https://www.sciencedirect.com/science/article/pii/S0041010106004363?via%3Dihub#aep-section-id30 Richard H. ffrench-Constant, Andrea Dowling, Nicholas R. Waterfield,Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture, Toxicon, Volume 49, Issue 4, 2007, Pages 436-451, ISSN 0041-0101,
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 [https://www.sciencedirect.com/science/article/pii/S0190962220329066 Robert Bissonnette, Linda Stein Gold, David S. Rubenstein, Anna M. Tallman, April Armstrong, Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor–modulating agent, Journal of the American Academy of Dermatology, Volume 84, Issue 4, 2021, Pages 1059-1067, ISSN 0190-9622,
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 [https://www.nature.com/articles/srep43670 Mouammine, A., Pages, S., Lanois, A. et al. An antimicrobial peptide-resistant minor subpopulation of Photorhabdus luminescens is responsible for virulence. Sci Rep 7, 43670 (2017).
- ↑ 6.0 6.1 6.2 6.3 6.4 [https://www.researchgate.net/profile/Ana-Antonello/publication/321191804_Entomopathogenic_bacteria_Photorhabdus_luminescens_as_drug_source_against_Leishmania_amazonensis/links/5a26b1884585155dd423ece7/Entomopathogenic-bacteria-Photorhabdus-luminescens-as-drug-source-against-Leishmania-amazonensis.pdfAntonello AM, Sartori T, Folmer Correa AP, Brandelli A, Heermann R, Rodrigues Júnior LC, Peres A, Romão PRT, DaSilva OS. Entomopathogenic bacteria Photorhabdus luminescens as drug source against Leishmania amazonensis
- ↑ 7.0 7.1 7.2 7.3 J, Gull K. Shape, form, function and Leishmania pathogenicity: from textbook descriptions to biological understanding [published correction appears in Open Biol. 2018 Aug;8(8):. Open Biol. 2017;7(9):170165.
- ↑ [https://pubmed.ncbi.nlm.nih.gov/11041562/Toescu EC, Verkhratsky A. Assessment of mitochondrial polarization status in living cells based on analysis of the spatial heterogeneity of rhodamine 123 fluorescence staining. Pflugers Arch. 2000 Oct;440(6):941-7.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2021, Kenyon College.
