Sense RNA Virus: West Nile Virus
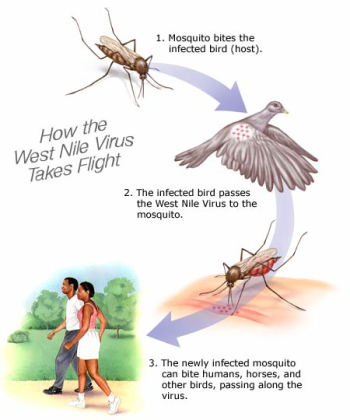
The family Flavivirdae has at least 68 viruses that have similar replication processes and are morphologically, morphogenetically, and biochemically similar. Flavivirdae fits into the category of an insect-transmitted vertebrate virus. The West Nile Virus is a species of the genus Flavivirus and in the Flavivirdae family. Other species include dengue virus, Tick-borne Encephalitis Virus, Yellow Fever Virus. Its main hosts include humans, birds, cows, dogs, cats, and other animals. Transmission of the virus occurs from the bite of an infected arthropod (i.e. a mosquito).
After genome sequencing, West Nile Virus (lineage II strain 956) was determined to have 10,962 nucleotides with a GC content of 51%, 93% of the RNA is coding, the topology is linear, and it is a ssRNA molecule.
These types of viruses cause encephalitis, which is an inflammation of the brain and spinal chord (myelitis). Symptoms of the West Nile Virus include fever, headache, chills, vomiting, and a rash. More serious symptoms include a loss of consciousness and spinal chord infection. The mortality rate has decreased greatly in the human population from 11% in 1999 to 3% in 2008. Most cases are mild enough where they are not reported. However, species of birds and cows see much higher mortality rates that range from 60-100%.
West Nile Virus was first studied in the West Nile District of Uganda in 1937, but its phylogenetic lineage presents that it became a separate virus about 1000 years ago. The spread of the Japanese encephalitis virus (JEV) lead to the first reports of the virus in the United States in 1999. Throughout the world, human populations have been plagued with outbreaks. Countries such as Romania in 1996, the Czech Republic in 1997, the Congo in 1998, and Israel in 2000 include more of the recent outbreaks.
Replication and Potential Drug Targets
Flavivirus are a group IV virus. They are (+) sense, single-stranded viruses. To infect the host cell the virus envelop must attach and fuse to the host cell. Through studies done by Gollins and Porterfild, evidence was presented to show that flaviviruses are absorbed by vertebrate cells through receptor-mediated endocytosis and then a pH-dependent fusion episode. Research has also shown that infection of West Nile could involve the fusion of the virus straight to the host cell’s plasma membrane. Once in the cell the single stranded RNA is able to go through transcription and translation. After the completion of translation a polyprotein is produced. The amino acid sequences of all flaviviruses are highly conserved next to the their polyprotein proteolytic sites (Ludwig, Iocono-Connor, 1992). Since the virus is so small it can not carry all the necessary enzymes and proteins to complete its replication processes. This is why both host and viral proteases are directly involved in viral protein processing. The limitation to polyprotein processing is that it has almost been strictly isolated to mammalian cell culture systems. The reason for this could have to do with the antigenic reactivity of West Nile could be changed in insects compared to mammals. Virus particles are mostly isolated from the endoplasmic reticulum. This is common of (+) ssRNA, such as poliovirus.
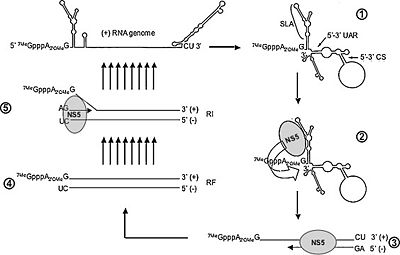
The (+) strand is the RNA strand that is translated directly to mRNA. The replication and translational process of the (+) sense strand and the mRNA is host dependent. Like many viruses that do not carry replication devices, Flavivirus requires RNA-dependent RNA polymerase to make a template for mRNA and genome replication. The host cells ribosome is need to translate the mRNA into a single polyprotein. The polyprotein includes structural and non-structural proteins. Within this polyprotein are mature polypeptide products that have catalytic as well as synthesizing functions. An important enzyme that is released after translation is responsible for cleaving a polymerase that synthesizes the (+) strand into a (-) sense RNA molecule. The template strand can then be synthesized by the RNA-dependent polymerase to create genomic progeny RNA. The genomic progeny is assembled into an enveloped, icosahedral nucleocapsid. This happens when the virus buds from the cell.
Currently there are no antiviral drugs available against any Flavivirus infection. Since Flavivirus depend on the RNA polymerase for replication of its genome, drugs are designed to target the binding of the RNA polymerase. Two structures provide initiation sites for the RNA polymerase: a priming loop and a zinc-ion binding site. In a study done by Helene Malet, et.al, the binding of the NS5 RNA-dependent RNA polymerase (RdRp) was tested for possible antiviral drugs. The 5’ and 3’ terminal regions of the Flavivirus RNA genome form complementary cyclization sequences. This structure is important for RNA replication. The RdRp domain binds to the promoter stem loop A at the 5’ end (SLA) of the genome and initiates RNA synthesis using the 3’ end as a template. The interaction between the RdRp domain and the SLA is another possible target. Another part of the process that is targeted for drug discovery is the changing conformation of the RdRp domain during the initiation and elongation phases of the RNA synthesis. The RdRp enzyme changes from a ‘closed’ conformation during initiation and an open conformation during elongation. Also different primers where seen to be present during initiation and elongation. An effective drug design would be to target and confine the enzyme responsible for initiation. The structure of the RdRp has a right-hand structure and contains three domains: fingers, palm and thumb. The fingertips and the thumb connect to form the priming loop and the active site is located in the palm.
Polymerase inhibitors fall into the categories of nucleoside analogue inhibitors (NI) and non-nucleoside analogue inhibitors (NNI). NIs target the active site of the polymerase and NNIs bind to allosteric surface cavities of the target polymerase (Malet, et.al, 2008). NNIs have been shown to be very successful at restraining HIV reverse transcriptase activity.
The E protein is on the surface of the flaviviral envelope and is considered an important part of the structure. On the envelope there are spikes which allow it to recognize the receptor on the host cell and attach to it. Once attached the virion is able to inject its genome into the host cell where replication occurs. The E protein also contains antigenic determinants responsible for hemagglutination and neutralization, so it is important in immunology protection from infection (Ludwig, Iacono-Connors, 1992). The E protein can be a target for antiviral drug design. Without the ability to attach to the host cell and prevent infection the virus would not be able to transfer its genome to the host.
Like many viruses the process of packaging the West Nile virion occurs at the same time as transcription and translation. The M protein which is important in the membrane protein structure and Flavivirus activation has two different states. The pre-M protein is present in most intracellular virions, which are in infected cells. This particle is said to be immature. The pre-M protein is then transported to the cell surface by the translocation of immature virion-containing vesicles. The vesicles and the plasma membrane then fuse and the virus’ genome is released extracellularly. At the same time amino particle that is on the end of the pre-M protein is released by a proteolytic cleavage process. This incident produces a mature M protein, which is necessary for an entirely infectious West Nile particle. This is the process that is pH dependent and occurs in post-golgi acid vesicles. Once outside the lysed cell the virion is ready to infect new host cells.
With no specific treatment for West Nile Virus, people who become severely infected are placed in hospitals. Mild cases of the virus are not treated and the symptoms are no longer present. In hospitals patients treatments include IV fluids and nutrition, breathing tubes, machines for breathing, and prevention of secondary infections.
Infection of Birds and Crows
The West Nile Virus is not isolated to just humans, it includes other species such as birds, cows, cats, and dogs. The virus is maintained through bird species and the mosquitoes that feed on them. Birds are an important natural host for the virus. The efficiency of carrying the virus is dependent on the species. From recent studies perching birds (Passeriformes), certain gulls (Charadriiformes), and two birds of prey species (Falconiformes and Strigiformes) are more likely to pass the virus on to mosquitoes. Mosquitoes that transfer the virus are called mosquito vectors. There are many kinds of species of mosquitoes, but not all have been tested positive for West Nile Virus. Once the virus has been transmitted to a human host, a mosquito that feeds of the host can not become a carrier due to the low concentration of the virus in the human blood. Some of the mosquitoes that carry the virus aren’t even able to transmit the virus to a human host. The animals listed are more affected by the virus than humans. Once infected, they have almost no chance of survival. The virus can diminish entire populations. With the westward and southward spread of the virus across the country, more populations were becoming infected. The first naturally occurring case of the virus in owls was found in Michigan in August of 2002. The identification of microscopic lesions that are present on animals like cows and birds were identified as part of the naturally occurring process during infection. A study done by S. D. Fitzgerald et.al in 2003 looked at the infection in Native North American Owls. The infected owls were hit with increased rates of neurologic disease and death. The owls were collected in both Michigan and Ohio. To test for West Nile Virus samples of the lung, heart, brain, liver, kidney, spleen, gastrointestinal tract, pancreas, and gonad were removed and examined for histopathologic and IHC examination (Fitzgerald, et.al, 2003). The owls that were infected showed uncoordinated flight, inability to fly, depression, head tilt, seizures, and death. The areas that showed the highest rates of microscopic lesions were the heart and the brain. The West Nile Virus attacks the tissues, so immunohistochemical (IHC) tests were taken to determine if the tissues of the organs were affected by the virus. Twelve out of the thirteen owls tested positive in more than one of the organs, and the heart and kidney tested positive more frequently than others. To determine virology RT-PCr and TapMan RT-PCR were used on the thirteen owl species. Eleven out of the thirteen owls showed positive results for West Nile virus RNA. With a Ct value they were indirectly able to figure out the viral RNA content that was in the tissues. The difference in infection is due to the difference in species of owls.
From the study of crow populations hypotheses were developed about infection and the transfer of West Nile Virus. During the months of June through October in 2003, scientists in the Zoology Department at Oklahoma State University observed an American Crow population in Stillwater Oklahoma. After the study was complete they estimated that 65% (51 of 78) individuals died due to infection of the West Nile Virus. Out of this population some of the dead crows were not found. This was due to the fact that dead crows are very easy to prey on. These carcasses could have been eaten or transported to new areas. Depending on the animal that ate the crow, it could have transmitted the disease. Crows can also become infected by eating prey such as other birds or mice as well as the bites of mosquitoes, drinking water containing viral particles, and physical contact with another infected crow (Komar, et al. 2003). However horizontal transmission of the virus was not supported. If crows had infected other crows there would have been a greater number of deaths in the following week (Komar). If the dead birds were transferred to a new location this presents the possibility of spreading the disease. In terms of prevention the isolation of the infected population is very important. In comparison to the owls, the tests done on infected crow populations throughout the United States, show similar infection sites. IHC staining showed that high concentrations of viral antigens were present in tissues from the heart, kidney, and brain. Owls show lower viral presence than crows, which could mean they are more resistant to infection as well as a longer disease course (Fitzgerald).
No matter how you look at it birds cover large areas of territory. This leads to the spread of the virus. Prevention methods are developed to control bird populations that have a high infection, transmission, and death rate.
Prevention
An important mode of prevention is to determine the frequency, patterns and concentrations of the virus in given populations of the carriers (insects) and the hosts (birds and mammals). To be able to study the virus the virus must be cultured in cells. Cell cultures of the virus are very important and can be done in either invertebrate or vertebrate cells. The development of isolations diagnostic techniques allow for cell and tissue culture systems to be analyzed. From these studies human and animal populations that are at risk can be properly and safely handled. The species of mosquito carrying the virus has an effect on how the virus is grown in culture. The development of cell lines to isolate a virus and diagnose infection has been very beneficial in the research of flavivirus. Cell lines have been obtained from A. albopictus, A. pseudoscutellaris, Toxorhynchites amboinesis, and others have been important in the study of West Nile Virus. Through the growth features of West Nile in culture the prime cell type, laboratory, temperature, and type of experiment have been determined.
In Response to the onset of West Nile Virus, the New York State Department of Health and other state agencies designed a plan for the prevention of the virus. The control strategy was designed by the Integrated Pest Management (IPM). Their main goal was to prevent the number of mosquitoes that carry the virus. To do this the most pursued method of controlling mosquito population was to target the mosquito larvae. While the state had control of population control in some areas, local homeowners were asked to help in the process. Part of the IPMs plan is to educate the homeowners and others on how to prevent the mosquito population. It is easier to control the populations of the larvae than mosquitoes because of the mobility of the mosquitoes. It is beneficial to the environment to have target specific agents in definable areas. The use of pesticides is harmful to the environment as well as humans, so applying the pesticides by ground instead of an aerial approach is preferred. A system of surveillance was designed called the Tier Circumstances Response Table. Through this table the prevention units were able to decide the best approach to killing the mosquitoes. There are many factors that must be assessed before pursuing an aerial approach: size of the vector population, the vectors physiologic use, the density and proximity of human populations, the time of year, weather conditions, access the vectors location, and likelihood of migration of a vector (New York State Department of Health).
Mosquito larvae are heterotrophs that begin their life cycle in standing water and eat organic materials. Based on the larvae’s ecological and metabolic characteristics the prevention methods targeted mosquito breeding grounds. The prevention methods of residential and commercial properties include removing standing water from pool covers, abandoned pools, rain gutters, and establishing a fish population in ponds, the federal, state, and local governments were in charge of existing drainage structures in government run stations. Microbial larvicides such as Bacilus thuringiensis var. israelensis and Bacilus sphaericus were used in many freshwater habitats. In salt water habitats biochemical larvicides such as methoprene were used.
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
[West Nile Virus (http://www.entomology.cornell.edu/MedEnt/WestNileFS/WestNileFS.html)-Cornell Cooperative Extension.] [Genome of West Nile Virus (http://www.ncbi.nlm.nih.gov/sites/entrez?db=genome&cmd=Retrieve&dopt=Overview&list_uids=10411)-NCBI]
