Bacillus Anthracis NEU2011
Higher Order Taxa
Domain: Bacteria
- Phylum: Firmicutes
- Class: Bacilli
- Order: Bacillales
- Family: Bacillaceae
- Genus: Bacillus
- Species: Bacillus anthracis (Cohn 1872)
Description and significance
Bacillus anthracis is a rod shaped bacteria with a width of 1-1.2 um and a length of 3-5 um. It can be grown in an ordinary nutrient broth, under either aerobic or anaerobic conditions. It was one of the first organisms to be proven to show disease, by Robert Koch. B. anthracis is similar in size, morphology, and spore formation with Bacillus cereus, and Bacillus thuringiensis.It is gram positive and is able to form spores. These spores are highly resistant and are able to sustain drastic changes in temperature, low nutrient environments, and application of harsh chemicals (including disinfectant chemicals such as 95% ethanol) over many years. Because the spores are so resilient, it is often hard to control this bacteria.4
B. anthracis is pathogenic and is associated with the disease Anthrax. Anthrax is mostly obtained by livestock, such as cattle, goats and sheep. This is because the bacteria in the soil often form spores, which last for many years. Then an animal comes along, grazing on the grass where the spore is located and ingests the spore. Once in the body, the spore germinates and begins to grow and divide. Humans can obtain three different types of anthrax, intestional anthrax, cutaneous anthrax, and inhalation anthrax.
Genome Structure
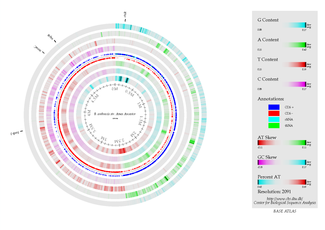
B. anthracis has one chromosome and its genome is 5.5 Mb in size. The chromosome contains 5,227,419 base pairs. Its virulence factors are encoded on two plasmids, pXO1 (anthrax toxin, 181,677-bp) and pXO2 (capsule genes, 94,830-bp).(12)
The B. anthracis genome is highly comparible to the organism Bacillus cereus, and the two are thought to reside in the same phylogenetic cluster. The main difference between the two is the toxicity. B. cereus has minimal toxic effects, while B. anthracis can cause very serious disease, which is mainly attributed to its two toxin plasmids which work in unison to cause disease in a host. One of the virulence plasmids, pXO1, encodes a toxin which produces either a lethal factor (LF) or an edema factor (EF), as well as a protective antigen (PA). These toxins work together to target a host, ultimately resulting in suppression of the immune system and eventually the death of the organism. The second plasmid, pXO2, carries genes for the production of a capsular polysaccharide (CPS). This polysaccharide works to protect B. anthracis from any immune response from the host. (12)
The first successful completion of the B. anthracis genome was published by The Institute for Genomic Research in 2003. Using whole-genome shotgun sequencing, a complete sequencing of the non-virulent strain (the strain lacking the two virulent plasmids) was created. Since then, TIGR has also sequenced the virulent strain. Currently, 6 sequencing projects for the B. anthracis genome are in progress and 5 projects have been completed. (12)
Ecology
Being part of the genus Bacillus, B.anthracis primarily inhabits slightly alkaline, calcium-rich soil and grows on decaying organic matter 4, 5. Upon favorable conditions, it exists in its natural rod-shaped form 4, 5. However, when the conditions become harsh, B. anthracis forms a spore using the calcium from the surrounding habitat for sporulation 4, 5. This spore is rigid and is resistant to disinfectants, heat and drying4, 5. Because of this resistant spore, B. anthracis is an extremely harmful pathogen that causes anthrax and is extremely difficult to treat 5. It can remain dormant for years until the conditions are appropriate for growth and reproduction, such as in an animal where the bacteria can use the animal as a host 5. This essentially classifies the B. anthracis as parasitic 5.
The organism can be contracted via contact with soil that has colonies of the microbe inhabiting it, or in pools of water where "B.anthracis" in spore form may be found 4, 5. The animals that are most prone to infection by B. anthracis are grazing herbivores such as sheep or cattle as well as other domesticated animals such as horses and mules 4, 5. All warm-blooded animals are susceptible to infection due to the fact that "B.anthracis" grows optimally at a temperature of 30-39 degrees celsius, covering the body temperature range of all warm-blooded animals4, 5. Therefore, because cold-blooded animals do not sustain this temperature range, their risk of being invaded by B. anthracis is very low 4, 5.
Cell Structure and Metabolism
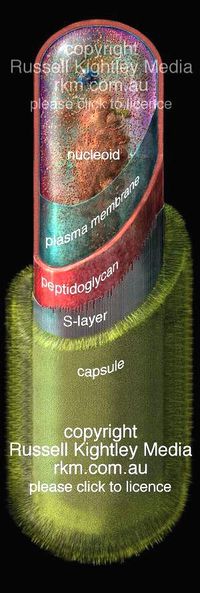
B. anthracis is a gram-positive bacteria, indicating a very bulky cell wall which usually consists of the thick peptidoglycan layer, teichoic acids, lipoteichoic acids, capsulare polysacchrides, and crystalline cell surface proteins (often refered to as the S-layer proteins) which are often glycosylated.2 B. anthracis is different from this typical description of a gram positive bacteria. It’s capsule poly-γ-D-glutamate instead of the usual polysaccharide capsule, it’s S-layer are not glycosylated and finally, it does not contain any teichoic acids. [1]
These differences could provide a evolutionary advantage for this bacteria, for example, polysaccharides are associated with the adhesion of alpha defensins which are secreted by neutrophils to kill bacteria. By not having these polysaccharides, in the capsule, and instead have it composed of poly-D-glutamic acid, B. anthracis is not affected by a neutrophilic attack, and are able to survive longer in the host without immunological intervention. This capsule is also important because it has a negative charge, which protects it from phagocytosis of macrophages.
The genomic material is organized into a ball of DNA and is condensed to be called the nucleoid. This is not a membrane bound organelle, but but consists of condensed amount of DNA. Some of these bacteria have an extra segement of DNA call a plasmid, which supplies extra genetic material that is not necessary for regular cell growth but often provides bacteria with helpful genomic material.
Other then the nucleoid, ribosomes are also present in the cytoplasm, these supply energy for the bacterial cell.
The genus Bacillus indicates bacteria that are rod shaped. B. anthracis is often found in short chains and have distinctive square ends. Also unique of B anthracis from other Ballius species, is that this Bacillus is pathogenic.
The bacteria is also aerobic, which means that it needs oxygen in order to be vegetative. Oxygen is also necessary for the formation of spores. In addition, B. anthracis does not have any flagella and is non motile.
Spores are the infection agent of Bacillus Anthracis. They are designed to withstand harsh environments until adequate surroundings and nutrients are available and then the spores germinate and become vegetative cells. B. anthracis forms into spores when there is a loss of nutrients, or another necessary element starts to go missing from the environment they currently live in. When there is a drop in nutrients and spore formation has to occur, a cascade of events involving sigma factors occur and as a result there is expression of mRNAs which are responsible for spore development.
Once a mature spore is formed, it can lay dormant for decades. These spores are completely metabolically inactive, and can withstand drastic temperature changes, along with exposure to chemicals and radiation. These spores will not germinate until signals are received via essential nutrients or vital ions in the presence of an aqueous environment, which cause a chemical change in the spores initiating germination.
Pathology
B. anthracis is the causative agent of the disease Anthrax, a disease affecting hoofed animals (sheep, goats) as well as humans. This disease most often involves the skin, gastrointestinal tract, and the lungs. Anthrax was used as a bioweapon in October 2011, when it was sent through the U.S. Postal Service as part of a bioterrorist attack. 22 people were infected by the bacterium, while 7 survived. Consequently, this attack led to more extensive research about the pathology and treatment of Anthrax.
This bacterium has the ability to infect new hosts in 3 ways: inhalation, ingestion, or through the skin (cutaneously). The patient may experience a wide range of symptoms based on the way in which their body encountered the bacterium. If B. anthracis is ingested, patients will likely experience chills, fever, shortness of breath, chest pain, and general discomfort or malaise. If the bacterium was ingested into the gastrointestinal tract, patients will present with nausea, vomiting, anemia, and bloody diarrhea. These symptoms usually develop within one week. As the disease progresses, patients will experience tachycardia (abnormally rapid heartbeat) and hypotension (abnormally low blood pressure) if it goes untreated. Symptoms of cutaneous antrax include blisters and ulcers surrounded by swelling. (9)
If the Antrhax disease is suspected, it can be tested by way of skin culturing, chest X-ray, sputum culture, spinal tap, and Gram staining. If a patient tests positive, they can be treated using a combination of antibiotics, taken either orally or intravenously. If Anthrax goes untreated, the patient will enter the second stage of the disease. Gastrointesinal anthrax infection as well as cutaneous infection can spread into the bloodstream and lead to a host of life-threatening complications such as shock, hemorrhaging, swelling of lymphnodes, and even death. With antibiotic treatment, the prognosis is often very good, especially if it is caught in the first stage. Up to 90% of cases with second-stage Anthrax are fatal. (9)
Current Research
Most current research on B. anthracis is actually focusing on the toxins it produces. Mostly it involves making small changes in the toxins and observing the result.
Cool Factor
The lethal factor of B. anthracis is a metalloprotease that is translocated into the cytosol by the protective antigen protein by binding to specific receptors on a macrophage cell. Once the lethal factor is inside the macrophage, it begins its destruction of cellular pathways. One of the most important pathways that the lethal factor inhibits is the MAPK pathway, which is essential for proper growth of the cell. The lethal factor cleaves the amino terminal end of the mitogen-activated protein kinase kinase (MKK) so that this enzyme becomes non-functional and the entire MAP kinase pathway halts. The MKK is responsible for p38 activation, which plays a role in quelling apoptosis. Therefore, if p38 is not activated by the MAP kinase pathway, the cell will undergo apoptosis. The death of the macrophages means that B. anthracis will be undetected because the macrophages will not be able to send cytokine and chemokine signals to alert the immune system of an infection. This is primarily why the infection will not be detected by the immune system until great bacteremia occurs 10.
References
[1] Anthrax: Bacillus anthracis by Russell Kightley Media. (n.d.). Russell Kightley Media: Scientific Illustrator (science illustration, graphics and animation) Home Page.. Retrieved April 28, 2011, from http://www.rkm.com.au/BACTERIA/anthrax.html
[2] Baron S (1986). Medical Microbiology. Menlo Park, CA: Addison-Wesley, Health Sciences Division. http://www.ncbi.nlm.nih.gov/books/NBK7699/#A927
[3] Choudhury B (2006). The Structure of the Major Cell Wall Polysaccharide of Bacillus Anthracis Is Species-specific. Journal of Biological Chemistry. 281(38): 27932-7941. http://www.jbc.org/content/281/38/27932.full
[4] Firoved A, G Miller (2005). Bacillus anthracis Edema Toxin Causes Extensive Tissue Lesions and Rapid Lethality in Mice. American Journal of Pathology. 167: 1309-1320. http://www.ncbi.nlm.nih.gov/pubmed/16251415
[5] Gardner R (2011). Anthrax (Bacillus Anthracis). CSA. ProQuest. Web. <http://www.csa.com/discoveryguides/anthrax/overview.php>.
[6] Hugh-Jones M, J Blackburn (2009). The ecology of Bacillus anthracis. Molecular Aspects of Medicine. 30(6): 356-367. http://www.ncbi.nlm.nih.gov/pubmed/19720074
[7] Inglesby TV, T O'Toole , DA Henderson (2002). Anthrax as a Biological Weapon. JAMA. 160(287): 2236-2252. http://jama.ama-assn.org/content/281/18/1735.full
[8] Kenneth T (2001). Bacillus Anthracis and Anthrax. Online Textbook of Bacteriology. Web. Feb. 2011. <http://www.textbookofbacteriology.net/Anthrax.html>.
[9] Lucey DR (2007). Anthrax. In: Goldman L, Ausiello D, eds. Cecil Medicine. 23rd ed. Philadelphia, Pa: Saunders Elsevier; chap 317. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0002301/
[10] Park JM, FR Greten, ZW Li, M Karin (2002). Macrophage Apoptosis by Anthrax Lethal Factor Through p38 MAP Kinase Inhibition. Science. 297(5589): 2048-2051. <http://www.sciencemag.org/content/297/5589/2048.full>
[11] SAMMD. (n.d.). SAMMD. Retrieved March 21, 2011, from http://pathogengenomics.net/
[12] Spencer RC (2003). Bacillus Anthracis. Journal of Clinical Pathology. 56(3): 182-187. http://jcp.bmjjournals.com/content/56/3/182.full
[13] TIGR. http://www.ncbi.nlm.nih.gov/genomes/MICROBES/anthracis.html
[14] Ussery, David W.. "DNA Atlas (Base Atlas) for Bacillus anthracis strain Ames, Main." Welcome to CBS. N.p., n.d. Web. 10 Apr. 2011. <http://www.cbs.dtu.dk>.
