Neisseria meningitidis causing meningococcal meningitis
Introduction

Neisseria meningitidis, gram-negative diplococci that cause meningococcal meningitis (magnified x 33000) (http://www.dailymail.co.uk/sciencetech/article-2197533/As-pretty-picture-lot-deadly--Killer-diseases-youve-seen-before.html).
By Kelsey McMurtry
Neisseria meningitidis is a gram-negative diplococci of the Proteobacteria phylum (7). This bacterium specifically causes disease in humans (1), and is one of the three main bacteria that causes acute bacterial meningitis, along with Streptococcus pneumoniae and Haemophilus influenzae. Of the forms of acute bacterial meningitis, Neisseria meningitidis causes meningococcal meningitis, which is among the top 10 causes of death due to infection across the globe, where one-third to half of people who survive the infection deal with permanent physical or mental side effects of the disease (2). These side effects, also known as sequelae, can include chronic fatigue and insomnia, which were symptoms found in those who survived epidemic meningococcal meningitis (2). In fact, these symptoms closely reflected those of individuals who experienced post-traumatic stress disorder following survival of septicaemia and septic shock (2). These two conditions involve the bacterium infiltrating the bloodstream, which is normally a sterile environment.
What is bacterial meningitis?
According to A. Chaudhuri, bacterial meningitis is defined as the "leptomeningeal inflammation resulting from infection of the pia" (2). This means that the layers that surround the central nervous system- the meninges- are inflamed. The meninges are composed of the of pia, the arachnoid, and the subarachnoid space (see figure). Many cases of meningitis involve the spread of bacteria from an infection in a completely different part of the body by traveling through the bloodstream to the brain and spinal cord. However, bacteria have been also known to spread throughout the body from a significant blow to the head, or from an infection initially located in the ear, nose, or teeth. Unfortunately, as the disease persists, the brain begins to swell and can possibly begin bleeding.
Metabolism
The plating of
Neisseria meningitidis on chocolate agar in order to determine if it truly causes meningococcal meningitis, suggests that it is able to use glucose as a form of energy. If this is in fact the case, then there is the possibility that it uses the EMP, ED, the PPS and/or the TCA cycle to form and utilize energy. In a study by Gino JE Bart and colleagues, they modeled by accessing the genome the metabolism of this bacterium. In their findings, they discovered that the bacteria mainly uses the PPS and the ED pathway to synthesize pyruvate into different forms of energy for the bacterium.
Pathophysiology of the disease
In an initial step, the host cell becomes infected by the bacteria in its attachment onto the cell by using a Trimeric Autotransporter Adhesins (TAA) (7). As a rapidly progressive disorder, meningococcal meningitis involves
Neisseria meningitidis invading the subarachnoid space of the brain, where the body responds with inflammation of the meninges due to bacterial infection. After the bacteria replicate in the bloodstream, they travel through the blood and sometimes cross the blood-brain barrier and take over the subarachnoid space. While in the bloodstream, white blood cells attack
Neisseria meningitidis, but unfortunately once the bacteria enter the subarachnoid space, the host's defense network is unable to control the infection developing in the cerebrospinal fluid (CSF) because the presence of safeguards (local antibodies and complement activity) is lacking in the area. As the numbers of bacteria and white blood cells increase in the CSF, a local inflammatory response is induced in the subarachnoid space due to the creating and releasing of inflammatory mediators. These nasty compounds cause an increase in the number of white blood cells within the CSF and increase the accessibility of the blood-brain barrier, a key ingredient to the bacterial meningitis infection. In response to the accessibility of a typically sterile environment to pathogens, mediators (including liposaccharides and cytotoxin-engaging receptors, including what are called Toll-like receptors) of endothelial cells activate downstream cascades that release white-blood cell precursors and other immune cell responses. This allows for white blood cells (specifically neutrophils, as shown in the figure) to move across the blood-brain barrier (6).
Although the host can show signs of symptoms such as confusion, stupor, coma, and seizures that correlate with bacterial meningitis, these conditions are not directly caused by bacterial invasion because the bacteria cannot invade the tissue located underneath the pia. Accessing the subpial tissue is a chemical response induced by proinflammatory cytokines, which are small signaling molecules used for cell signaling, that are released in response to the bacterial invasion and the breaking down of cells within the subarachnoid space (please refer to the figure for further explanation and understanding).
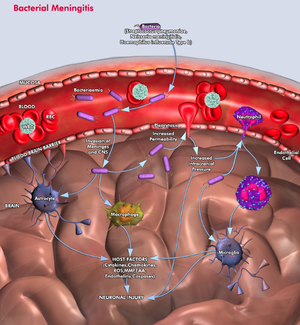
Neisseria meningitidis invades the meninges (http://www.qiagen.com/geneglobe/static/images/Pathways/Bacterial%20Meningitis.jpg).
Where, Who, What?
Neisseria meningitidis has the greatest outbreak potential of the three bacteria causing acute bacterial meningitis, and regularly causes outbreaks in the "meningitis belt" of sub-Saharan Africa (where epidemics occur every 8-12 years, and it is particularly critical in this area). Overall, as the cause of meningococcal meningitis, Neisseria meningitidis affects a variety of forms (or serotypes, as will be discussed below), and affects a large range of ages, but is found most frequently in children and those of college age (2).
Children and young adults are especially at risk for contracting meningococcal meningitis, especially with its ability to cause the spread of infection not just throughout the bloodstream, but also in the organs, also known as sepsis, as well as well as its ability to invade the typically bacteria-free environment of the blood (1). Particularly when looking at young adults, college students seem to be particularly at risk due to living in close quarters with many other individuals with diverse backgrounds, and where when stress levels rise and the amount of sleep decreases, so too does the susceptibility to infection. The immune system's ability to fight off infection may be due to a number of activities that college students engage in, including drinking, partying at clubs, smoking, etc... Unfortunately, every year, 15 to 20 college students die due to bacterial meningitis (5). And because there are varying symptoms for the disease, it is difficult to diagnose meningitis in the early stages of the infection (3).
In terms of case severity, the side effects of meningococcal meningitidis, including sepsis (as previously described above), which occurs when an infection spreads throughout the whole body, and not just the blood, but also the organs, as well as hypotension, the condition of having abnormally low blood pressure, are actually more severe than the disease itself (1).
Symptoms vary from person to person, but can include high fever, stiff neck, headaches, rash, fatigue, confusion, and sensitivity to light.
Unfortunately for those contracting the disease, acute bacterial meningitis can cause disability and death within one day of such contraction.
Variety in Neisseria meningitidis
The meningococcal meningitis-causing bacteria has 12 serogroups, or forms distinguished by a possessing the same set of antigens on their cell membrane (where antigens are located on the cell membrane surface, and promote an immune response). However, most cases of meningitis are caused by the following 6 serogroups: A, B, C, W135, X, and Y (6). To carry the disease, bacteria lacking capsules are usually involved.
Serogroup B infection is the most common form of the disease in countries across Europe and North America, and unfortunately cannot be treated by either conjugate capsular polysaccharide vaccines or outer membrane vesicle vaccines that target epidemic disease caused by a single clone of the bacterium (3).
Epidemics are found to be mainly associated with serogroup A meningococci. However, there have also been outbreaks correlated with serogroup C, and most recently, within the past decade, serogroups W135 and X (1).
The distribution of the disease, although peaking in infants and adolescents, also differs by serogroup and in epidemics. The potential of an epidemic has been documented in all serogroups, but particularly in serogroup A, W135 and B. The ability to detect acute bacterial meningitidis in the lab creates concerns in terms of accuracy and detail, where the disease requires specific medi and fast processing to be detected. However, it is promising that detection has been helped with the use of molecular methods (please elaborate on this detail) (1).
Serogroup C and serogroup B are found most commonly to cause meningococcal meningitis in adolescents and adults Canada, the United States, and Europe.
N. meningitidis serogroups B and C are composed of polysialic acid, and they only vary slightly in terms of their structure (specifics in (10)).
Carrying, Identifying, Diagnosing, and Treating Meningococcal Meningitis
Interestingly enough, about 1 in every 10 people carries meningococcus in their throat and nose, where it is in a harmless state. During a meningitis outbreak, approximately 95% of people will carry meningococcus, with 1% of the population actually contracting the disease, as fortunately many are resistant to its' deadly throes (5).
In order for the bacteria to be transferred, if someone has the disease and shares their respiratory fluids with your own, such as through kissing, or the sharing of a drink, etc..., then it is very likely that you will contract the disease.
Identifying those individuals with meningococcal meningitis caused by Neisseria meningitidis, there must be access to hospital care, specific criteria for performing lumbar punctures, as well as access to proper laboratory techniques, which are especially hard for middle- and lower-income countires to access such resources (1).
To diagnose acute bacterial meningitis, the bacteria must be isolated from sterile body fluid (this is where the lumbar puncture comes in to play). Once a cerebrospinal fluid sample (/specimen) is obtained, the sample is cultured on a chocolate agar plate (look exactly at what the organism metabolizes, and how this works in the body) (7).
In order to be entirely certain that an individual has contracted acute bacterial meningitis, a lumbar puncture, also known as a spinal tap, must be performed. In this procedure, a needle is placed through the lower back and into the spinal cord, where spinal fluid is collected to determine if the meninges are truly inflamed as a result of bacteria (or in some cases viral) meningitis (5).
Because the disease needs to treated as soon as possible, as it attacks with full force without warning, treatment with antibiotics needs to begin immediately. In a review of autopsy data, many deaths caused by N. meningitidis occurred within 12-24 hours of the onset of symptoms. After an individual has been treated with antibiotics, inflammation occurs because the bacteria are being broken down. In order to take care of the inflammatory response, corticosteroids are sometimes used, which also help to decrease the amount of swelling occurring in the brain and the increased pressure within the skull (6).
To administer antibiotics, there are three things that should be accounted for: the trends in certain areas/regions in terms of health and disease, the age of the patient being treated, and if any other factors will affect the ability of the patient to be treated (if they have other health complications, cancer, etc...) (8).
There has been a sufficient lack of randomized studies used to evaluate treatment strategies, especially in lower-income countries.
Additionally, the progress of discoveries allowing for the treatment of acute bacterial meningitis seem only to help those of/in higher-income areas, where those in lower-income areas are suffering in terms of their available medical resources. There needs to be a much higher prevalence of affordable alternatives, especially for those lacking the resources to be treated by expensive vaccines (4).
Progress and challenges in acute bacterial meningitis
Unfortunately, the diversity of global patterns of meningococcal disease is greater because of the higher likelihood of an epidemic. For those that survive the disease, they are fortunate in that meningococcal has a lower risk than the other two bacteria that cause acute bacterial meningitis (Streptococcus pneuomoniae and Haemophilus influenzae). When looking at cases of meningococcal meningitis across the globe, Africa records the highest incidence of the disease with greater than 100 (endemic, where the disease is found only in particular location or region), and greater than 1000 (epidemic, when there is an outbreak of a new disease that affects an abnormally large amount of the population) per 100,000 people in a population per year. The lowest incidence region is Europe with 1-2 (endemic) and 2-10 (epidemic) per 100,000 people per year. Fortunately, no deaths have been reported (2012), but in terms of morbidity, 7% of survivors experience major long-term sequelae, as discussed above (in the Introduction).
With the mortality rate remaining relatively high despite advances in treatment of acute bacterial meningitis, there are several issues that complicate the medical community’s ability to diagnose and treat the disease. For one, because meningococci can easily circumvent the body's response to the infection by the exchange of genetic material, there have been individuals who are fearful that meningococci (as well as pneumococci) could replace their usual serotypes by responding to a conjugate vaccination, particularly where there is a significant number of individuals with the disease/a significant proportion of the population that has contracted the disease, and it is furthermore easily transferrable (4,1). Lack of surveillance in low-income countries allows for a higher prevalence of the deadly infection.
The length of time that protection is given by a vaccine, as well as the vaccine's "ability" to be immune to an infection, or particular antibodies, while an individual is exposed to it, has led to a lot of controversy in terms of treating acute bacterial meningitis (4). There has been a lot of discussion regarding the need for additional booster doses for conjugate meningococcal vaccines, as their has been evidence that serogroup B antibody concentrations decrease significantly for children provided their first dose at (and after) 1 year of being vaccinated (1).
According to D. Van De Beek and colleagues, there are two particular issues relating to bacterial meningitis (8,3). One of the issues pertains to making sure that the delivery of the particular drug (vaccine) is as effective as possible, in terms of cost, amount of material required, the reactivity, etc... Secondly, the effectiveness of the antibiotics administered to a given patient is very important. The given antibiotic must penetrate the blood-brain barrier in order for it to be a successful treatment. This administration is according to how much the blood-brain barrier was disrupted by inflammation (see the section regarding pathophysiology above), as well as the size, charge, interaction with the membrane, ability to bind with proteins, and the interaction with pumps taking material outside the body (8,3). The clinical effectiveness of the antibiotic is furthermore dependent upon the antibiotic cerebrospinal fluid concentration and its ability to combat the bacteria that caused the infection. Furthermore, the antibiotics require a proper amount of time to eradicate all of the bacteria that caused the infection to, more importantly, prevent the disease from recurring (8,3). However, it is hard to predict the timescale to use, depending on the bacteria causing the disease (as mentioned before, there are other bacteria besides Neisseria meningitidis that cause acute bacterial meningitis), how severe the disease is, in addition to the antimicrobial agent used. In high-income countries, many authorities recommend a minimum of 7 days of treatment for meningococcal meningitis.
Designing protein-based meningococcal vaccines is difficult due to the increased amount of genetic and antigenic diversity of Neisseria meningitidis. Because this bacterium is readily able to allow for genetic transformation and recombination, it has a variable population structure that reflects its accumulated mutations and horizontal genetic exchange. Therefore, there is a lot of variability in terms of the virulence of the meningococci, and thus there needs to be a vaccine that allows for complete coverage of both “hyperinvasive” bacterial lineages, as well as those bacteria who are commensalists (11).
Discrepancies in treatment
Based on available resources
Meningococcal meningitis has a case-fatality rate of 7% in high-income countries, but unfortunately in low-income countries, fatality can reach 50%. High-income countries have a history of long-lasting outbreaks of serogroup A of the disease, however; within the past 30 years, serogroup B outbreaks have been shown to occur in Norway and New Zealand (4).
Based on genetic factors
Genetic factors are important in determining an the possibility of an individual contracting acute bacterial meningitis, whether it be pneumococcal (caused by Streptococcus pneumoniae) or meningococcal (caused by Neisseria meningitidis).
Conjugate versus Polysaccharide Vaccines
Current Treatment Options
Many countries choose Penicillin G to treat meningococcal meningitis, especially when the diagnosis has determined that bacteria are causing issues (bacteriological). However, recently, N. meningitidis has become more resistant to penicillin over the past 20 years, because of changes in the structure of a penicillin-binding protein (PBP2), which is encoded by the penA gene. The different structures of the penicilllin-binding protein are likely to be due to transformation between N. meningitidis and non-disease-causing Neisseria species (this idea was discussed earlier, please see above, and perhaps condense this thought) (9). Changes made in PBP2 causes decreases in the ability of PBP2 to bind to penicillin G, and furthermore caused modifications in the peptidoglycan structure of the bacteria’s cell wall.
N. meningitidis is still susceptible to cefotaxime, chlroamphenicol, and ciprofloxin, which are third-generation cephalosporin antibiotics (7).
Vaccinations for Prevention of the Disease
In the United States, there are three vaccines available for the prevention of disease for those individuals 2 years of age and older.
References
(6) "Bacterial Meningitis." Qiagen: Sample & Assay Technologies. 2013. Web. 22 Apr. 2013. <http://www.qiagen.com/products/genes%20and%20pathways/Pathway%20Details.aspx?pwid=50>.
(9) Bertrand, Sophie, Francoise Carion, Rene Wintjens, Vanessa Mathys, and Raymond Vanhoof. "American Society for MicrobiologyAntimicrobial Agents and Chemotherapy." Evolutionary Changes in Antimicrobial Resistance of Invasive Neisseria Meningitidis Isolates in Belgium from 2000 to 2010: Increasing Prevalence of Penicillin Nonsusceptibility. Web. 22 Apr. 2013. <http://aac.asm.org/content/56/5/2268.full.pdf html>.
(2) Chaudhuri, A. "Adjunctive Dexamethasone Treatment in Acute Bacterial Meningitis." The Lancet Neurology 3.1 (2004): 54-62. Print.
(11) Feavers, Ian M., and Mariagrazia Pizza. "Meningococcal Protein Antigens and Vaccines." Elsevier (2009): B42-50. ScienceDirect. Web. 15 Apr. 2013. <www.elsevier.com/locate/vaccine>.
(3) Johnson, Steven, Lionel Tan, Stijn Van Der Veen, Joseph Caesar, Elena Goicoechea De Jorge, Rachel J. Harding, Xilian Bai, Rachel M. Exley, Philip N. Ward, Nicola Ruivo, Kaushali Trivedi, Elspeth Cumber, Rhian Jones, Luke Newham, David Staunton, Rafael Ufret-Vicenty, Ray Borrow, Matthew C. Pickering, Susan M. Lea, and Christoph M. Tang. "Design and Evaluation of Meningococcal Vaccines through Structure-Based Modification of Host and Pathogen Molecules." PLOS Pathogens 8.10 (2012): 1-13. PLOS. Web. 16 Apr. 2013. <www.plospathogens.org>.
(1) McIntyre, Peter B., Katherine L. O'Brien, Brian Greenwood, and Diederik Van De Beek. "Effect of Vaccines on Bacterial Meningitis Worldwide." The Lancet 380 (2012): 1703-711. Print.
(7)"Neisseria Meningitidis." Wikipedia. Wikimedia Foundation, 22 Apr. 2013. Web. 22 Apr. 2013. <http://en.wikipedia.org/wiki/Neisseria_meningitidis>.
(10) Peterson, D. C., G. Arakere, J. Vionnet, P. C. McCarthy, and W. F. Vann. "Characterization and Acceptor Preference of a Soluble Meningococcal Group C Polysialyltransferase." Journal of Bacteriology 193.7 (2011): 1576-582. Print.
(4) "Progress and Challenges in Bacterial Meningitis." The Lancet 380 (2012): 1623-624. Web. 15 Apr. 2013. <www.thelancet.com>.
(8) Van De Beek, Diederik, Matthijs C. Brouwer, Guy E. Thwaites, and Allan R. Tunkel. "Advances in Treatment of Bacterial Meningitis." The Lancet 380 (2012): 1693-702. 10 Nov. 2012. Web. 14 Apr. 2013.
(5) "What Are the Facts About Meningitis?" Meningitis Symptoms. Web. 13 Apr. 2013. <http://www.chacha.com/gallery/4869/what-are-the-facts-about-meningitis/46227>.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
