Efficacy of vaccines against Streptococcus pneumoniae
Introduction
Streptococcus pneumoniae is a pathogenic, gram-positive, α-hemolytic, anaerobic bacterium (Slonczewski and Foster, 2009). It causes pneumonia along with other pneumococcal infections some of which include bacterial meningitis, sinusitis, and otitis media (Pletz et. al. 2008; Slonczewski and Foster, 2009; Siemieniuk et. al. 2011). S. pneumoniae has developed antibiotic resistance to traditional β-Lactam drugs such as Penicillin (and its derivatives), which are a class of antibiotics that contain a β-Lactam ring in their molecular structure. Antibiotic resistance is acquired by alteration of targeted penicillin binding proteins (PBPs) in the resistant strains that lower its affinity to bind to penicillin (Slonczewski and Foster, 2009). Genome sequencing of the penicillin resistant strains show that point mutations in one of its penicillin binding proteins,PBP2x, decreases its affinity to bind to penicillin (Laible and Hakenbeck, 1991).
Due to the increasing rate of antibiotic resistance it is important to study newer ways to inhibit S.pneumoniae growth. This is because antibiotics select for growth of rare microorganisms in a population that is otherwise susceptible to the drug (Slonczewski and Foster, 2009). Antibiotic resistance can be overcome by developing vaccines that target virulence factors on the surface of S. pneumoniae thereby disabling the bacteria (Jedrzejas, 2007). Vaccines work by giving the organism immunity against infection by a particular pathogen. Vaccines contain a weakened or dead derivative of the pathogen and in its altered state vaccine pathogens are typically safe and unable to cause disease (WHO, 2013; NIAID, 2011). The non-virulent pathogens in the vaccine stimulate the organisms B cells to make antibodies for the particular antigens thereby developing immunity to that pathogen (NIAID, 2011). Subsequently, Memory T cells, which are antigen specific T cells, get stimulated upon re-exposure to cognate antigen and rapidly multiply thereby providing “memory” to the body’s immune system (NIAID, 2011). In addition to the weakened or dead form of bacteria or virus, the vaccine also contains antibiotics or preservatives to protect the body against any germs that might get into the vaccine (NIAID, 2008).
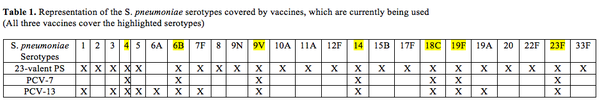
Current pneumococcal vaccines target the polysaccharide capsule of S. pneumoniae because immunity after pneumococcal disease is directed against the capsular serotype of the S. pneumoniae bacteria involved (WHO, 1999). Serotype is a characteristic set of antigens that help to distinguish a group of closely related microorganisms. 91 serotypes of S. pneumoniae have been identified based on the difference in composition of the polysaccharide capsule (Pletz et. al. 2008). The polysaccharide capsule is a virulence factor however; only some of the 91 serotypes cause pneumococcal disease. Current pneumococcal vaccines, Polysaccharide (23-valent PS) vaccine and conjugate vaccine (PCV-7) cover the most common and virulent serotypes and give immunity against 23 and 7 serotypes respectively. However, given the large number of serotypes (~91 serotypes), serotype replacement, which is the replacement of vaccine serotype (VTs) by non-vaccine serotypes (NVTs) in disease and carriage has become a growing concern thereby making vaccination against current serotypes less effective (Bogaert et. al. 2004; Pletz et. al. 2008).
Current pneumococcal vaccines
As an alternative to β-lactam antibiotics, vaccines have been created that target cell surface polysaccharide, which aim to develop immunity against the virulent serotypes of S. pneumoniae. The two vaccines widely used are polysaccharide vaccine (23-valent polysaccharide vaccine) and pneumococcal conjugate vaccine (heptavalent protein–polysaccharide conjugate vaccine, PCV-7) (Bogaert et. al. 2004). As the name suggests, the polysaccharide vaccine provides immunity against 23 S. pneumoniae serotypes and is effective in older immunocompetent patients (Bogaert et. al. 2004; Pletz et. al. 2008). However, this vaccine cannot be administered to infants below 2 years of age and to immunodeficient patients perhaps due to an immature or a sensitive immune system (Pletz et. al. 2008). The newer conjugate vaccine, PCV-7 is however able to provide immunity to this age group (Bogaert et al. 2004; Pletz et. al. 2008).
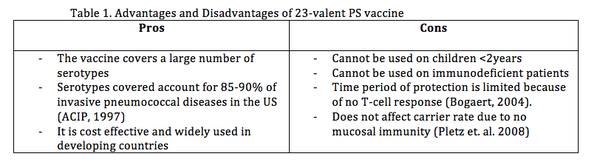
23-valent polysaccharide (PS) vaccine
The 23-valent PS vaccine contains capsular polysaccharide antigens for 23 serotypes and to gain immunity the polysaccharides induce B cell dependant response by releasing polysaccharide Immunoglobulin M (IgM), which are a type of antibodies produced by B cells (Pletz et. al. 2008). This PS vaccine does not induce a T cell-dependant immune response. As mentioned above, T cells provide ‘memory’ to the body’s immune system (NIAID, 2011) so antibodies can be made quickly upon re-exposure to cognate antigen. Also this vaccine cannot be administered to immunodeficient patients (Table 1), HIV infected individuals have show an impaired antibody response to PS vaccination (Kroon et. al. 1999). Further advantages and disadvantages are listed in (Table 1.)
A study done in Mexico evaluating the immune response of this vaccine in children under 5, for 6 serotypes, suggests that the pneumococcal polysaccharide vaccine produced adequate immunogenicity in the given age group (Padilla et. al. 2012). However, this vaccine is not effective in children under 2 years of age (Black et. al. 2000; pedilla et. al. 2012). Therefore, a more comprehensive vaccine is still needed.
Heptavalent protein–polysaccharide conjugate vaccine (PCV-7)
Unlike the 23-valent polysaccharide vaccine, which can only be used in children over 2 years the conjugate vaccine can be administered to infants as young as 2 months old (Bogaert et. al. 2004; Morsczeck et al. 2007; Pletz et. al. 2008). Polysaccharides in this vaccine are from seven serotypes (4, 6B, 9V, 14, 18C, 19F and 23F) that are most frequently involved in infant infections (Pletz et. al. 2008; Johanna et. al. 2011). As the name suggests this vaccine is conjugated to protein (CRM197), which is a non-toxic diphtheria toxoid protein (Pletz et. al. 2008). The protein-specific type 2 T cells associate with B cells, which are bound to the polysaccharide-protein complex via a polysaccharide specific IgM (Pletz et. al. 2008). This association between T and B cells presents the processed protein (CRM197) along with class II MHC (Major Histocompatibility Complex) to the effector T cells (Pletz et. al. 2008). Class II MHCs are cell-surface molecules that mediate interaction between immune cells and body cells. The antigens from Class II peptides come from extracellular proteins instead of from inside the cell. This mechanism leads to adaptive immunity in infants, which is switching of antibody isotype and generation of memory B-cells (Pletz et. al. 2008).
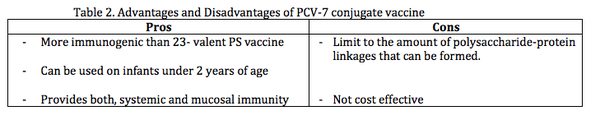
Pros and Cons for PCV-7
Pros
- More immunogenic than PS vaccine because capsular polysaccharides are covalently bound (or via reactive groups) to proteins carriers, which increases the immunogenicity of the capsular polysaccharides (Poland, 1999).
- The bound protein, CRM197 helps in eliciting a T cell dependent response, which induces memory of B cells (Bogaert et. al.2004).
- Can be used for infants under 2 years of age because of early maturation of T cell dependent response compared to the late maturation of antipolysaccharide antibodies (Bogaert et. al. 2004).
- Affects carrier rate by decreasing nasopharyngeal carriage of vaccine serotype, which suggests that conjugate vaccine provides both, systemic (throughout the body) and mucosal immunity (Spratt and Greenwood, 2000; Bogaert et. al. 2004). Obtaining specific mucosal immunity is important because it allows asymptomatic carriers to eliminate colonizing of vaccine serotypes thereby decreasing carrier rate (Pletz et. al. 2008).
Cons
- There is a limit to the amount of polysaccharide-protein linkages that can be formed. If too many carrier antigens are used antibody response to the antigens can be impaired (Di et. al. 1989).
- Not as cost effective because it is expensive to develop and does not cover the major serotypes (1 and 5) that cause pneumococcal diseases in developing countries (Morsczeck et al 2007; Jefferies et. al. 2011)
Combined use of 23-Valent PS and PCV-7
After the newer PCV-7 vaccine was developed, the best form of immunity was seen in patients that took the conjugate vaccine followed by the PS vaccine (Bogaert et. al.2004). A combined vaccination program was recommended for children 2-5 years by the ACIP, which recommended the use of PCV-7 first followed by the use of PS vaccine as a booster (Bogaert et. al.2004). This is perhaps because PCV-7 confers immunity in children from the invasive serotypes and later the PS vaccine provides immunity against additional serotypes not covered by PCV-7.
Serotype Replacement
Although antibiotic resistance has been a growing concern, use of vaccines against the major virulent serotypes has giving rise to a new problem that is serotype replacement. Serotype replacement is the processes in which pre-existing clones of non-vaccine serotypes (NVTs) can multiply and essentially replace the serotypes for which there is vaccination (Bogaert et. al. 2004). Replacement occurs because vaccines eradicate particular serotypes, this creates a niche for non-vaccine serotypes, allowing them to proliferation (Bogaert et. al. 2004; Pletz et. al. 2008).
Increase in non-vaccine serotypes (NVTs)
Studies have shown that with the administration of conjugate vaccine combined with re-immunization by 23-valent pneumococcal PS vaccine, reduces the prevalence of nasopharyngeal carriage of vaccine serotypes i.e. give mucosal immunity (Spratt and Greenwood, 2000). However, there has not been any significant reduction in the frequency of pneumococcal carriage because of ‘near-complete’ replacement with pneumococci of NVTs (Spratt and Greenwood, 2000; Weinberger, 2012). Beall et. al. 2006 genotyped invasive isolates, which were collected from patients after the administration of vaccine and compared it to isolates collected from patients that had not been administered PCV7 (Beall et. al. 2006). They found that in the non-PCV7 serogroup emergence of new serotypes was uncommon however, there was a significant increase in the proliferation of the ‘pre-existing clones’ of non-vaccine serotypes (Beall et. al. 2006). These results suggests that increase in NVTs is not due to emergence of new serotypes but instead is from the expansion of already established serotypes from which the vaccine gives no immunity.
Near-complete replacement in carriage Vs. Partial replacement for disease
There has been no significant decrease in the carriage of S. pneumonia because of the increase in the prevalence of NVTs in asymptotic carriers (Weinberger, 2012). As mentioned above, Beall et. al. 2006 found that vaccinated serogroups saw an increase of the pre existing NVTs. Randomized trials in South Africa, Netherlands and The Gambia show that NVTs have almost completely replaced vaccinated serotypes in carriage (Weinberg 2012; Beall et. al. 2006; Spratt and Greenwood, 2000). Also, presence of NVTs like 19A have led to an increase in diseased caused by NVTs (Vestrheim et. al. 2010). Study done by Vestrheim et. al. 2010 on the total Norwegian population from 1989-2008 suggests that once the conjugate vaccine was administered in 2006 the rate of invasive pneumococcal disease (IPD) caused by NVTs 19A, 9N, 33F and 35B in children under 5 increased (Vestrheim et. al. 2010). Moreover, IPD caused by 19A increased till 2007, after the conjugate vaccine was administered, suggesting that the increase in IPD by NVTs is due to serotype replacement and not due to an unrelated trend (Vestrheim et. al. 2010). However, the increase in IPD by NVTs has been modest and the rate of NVT replacement in carriage far exceeds the replacement in pneumococcal diseases caused by NVTs (Weinberger et. al. 2012).
The discrepancy in NVTs completely replacing carriage but only somewhat replacing for IPD is due to a combination of various factors.
• Difference in microbiological properties such as varying capsular size, adhesion, toxins and proteins of serotypes play a role in influencing carriage and invasiveness (Weinberger et. al. 2012). Serotypes that persist in carriage (NVTs) after vaccination tend to be heavily capsulated (Weinberger et. al. 2009).
• NVTs are less invasive than VTs therefore they are present in carriage form but do not cause pneumococcal disease (Weinberger et. al. 2012).
• Effect of ‘unmasking’ of the NVTs due to the reduction in VTs post vaccination, meaning that it is easier to detect new serotypes post-vaccination than when vaccine serotypes were present (Weinberger et. al. 2012).
Current ways to prevent Serotype Replacement
- Regularly monitoring and developing new conjugate vaccine
13-valent conjugated pneumococcal vaccine (PCV 13) is the newer version of PCV7 conjugate vaccine and contains a broader coverage range. It consists of thirteen pneumococcal capsular polysaccharides, which are individually conjugated to the diphtheria-derived protein carrier CRM (197) (Jefferies et. al. 2011). PCV 13 includes serotypes 4, 6B, 9V, 14, 18C, 19F, 23F1, 3, 5, 6A, 7F and 19A and will replace PCV7 particularly because it includes serotype 19A, which was a virulent NVT. In developing countries especially, it is a more cost effective option because unlike PCV7, it also covers for serotypes 1 and 5, which cause majority of pneumococcal diseases in developing countries (Jefferies et. al. 2011). Although PCV-13 is a better version of PCV-7 it is not antigenically covered across all serotypes a newer form of this vaccine might be required to address diseases caused by NVT.
- Identification of serotype independent antigens
Pneumococcal surface proteins, which have serotype-independence, can be used as alternative vaccine candidates (Bogaert et. al. 2004). Current pneumococcal vaccines fail to cover all polysaccharide types and infections in adults, particularly is caused by strains that have a variety of capsular types (Morsczeck et. al. 2007). Therefore it is necessary to developed alternative serotype independent vaccines.
Various cell surface proteins have been tested for as potential vaccine candidates however the most probable vaccine candidates are Pneumococcal surface protein A (PspA), Pneumococcal surface adhesin A (PsaA) and Pneumolysin. PspA is a protein virulence factor whose N-terminal domain is surface exposed and it is a member of choline-binding surface proteins (Bogaert et. al. 2004; Jedrzejas, 2007). PspA seems to be specific for bacteria producing choline residues on their surfaces (Jedrzejas, 2007). PsaA is a metal binding lipoprotein and part of the ABC transporter complex, which is involved in transport of manganese into pneumococci (Dintilhac et. al. 1997). PsaA has shown to be highly effective in mice models and a combination of PsaA and PspA also gave promising results in mice because PsaA and PspA have different functions in virulence (Bogaert et. al. 2004). Pneumolysin is also a virulence factor from S. pneumoniae; it is part of the thiol-activated cytolysin family (Jamie et. al. 1998). Pneumolysin has a choline-binding domain and one way by which it seems to interfere with host immunity and inflammatory responses is by inhibiting phagocyte function (Bogaert, 2004).
More recent research has used whole genome approach to detect protein-based pneumococcal vaccines (Morsczeck et.al. 2007). In a study done, Morsczeck et.al. (2007) used proteomics to identify cell-wall associated proteins, which were screened for vaccine candidates. Further immunization experiments on the 5 selected protein candidates; expressed in E.Coli revealed proteins that were detected in 40 or more different serotypes of S. pneumoniae (Morsczeck et.al. 2007). This study has selected 2 of the 5 candidates that are lipoate protein ligase (Lpl) and the ClpP protease that have shown a decrease in CFU count and should be used for further investigation.
Developing alternative protein vaccines seems like a likely next step to reduce the likelihood of growth of S. pneumoniae. Although the problem of antibiotic resistance and replacement is growing, targeting common denominators across different serotypes will help in eliminating the different strains of S. pneumoniae. However, the disadvantage with using immunogenic proteins is that in vivo they might not work as well. This is because antibodies might not be able to bind to targeted proteins in the cell wall that lie under the capsule polysaccharides (Pletz et. al. 2008).
References
Poland GA. 1999. The burden of pneumococcal disease: the role of conjugate vaccines. Vaccine
Slonczewski, J.L and Foster J. 2009. Microbiology. 2nd. W.W.Norton and Company.
World Health Organization. 1999. Pneumococcal vaccines. Weekly Epidemiological Record