Rabies
Taxonomy
| Order = Mononegavirales | Family = Rhabdoviridae | Genus = Lyssavirus | species = Rabies Virus| [1]
|
NCBI: [6] Genome: Rabies Virus [7] |
Description
Rabies (derived from Latin word Rabere, meaning 'Rage') is a viral disease caused by Lyssavirus rabies virus.[2] It is a neurotropic virus, capable of invasive infection of the central nervous system. The disease is extremely lethal to those unfortunate enough to show symptoms of the infection. The primary transmission of the virus to humans occurs through dog bites and scratches. The numbers vary widely form country to country, however, it is estimated that 55,000 people die per year from the rabies virus in Asia and Africa . The disease can be dramatic in its appearance as with many animals the disease is known to cause hyperactivity and sporadic behavior while becoming aggressive to things around them and eventually succumbing to paralysis of muscles.[3]
History and Impact

Rabies is a very old disease having originated before much of written history. The relationship between dogs and humans is shaped and altered greatly by the this disease originating from mad and crazed animals. Some of the oldest texts that are linked to rabies come from 2300 BC Eshunna, a Babylonian city, known for heavily fining people who's dogs bit others and lead to their death. Through out the Greek and Roman Empires rabies was well known and even widespread. The Greek philosophers even wrote that "dogs suffer from the madness." (Aristotle 400B.C.). It was the Romans who first described the saliva from rabid dogs as 'virus' which is Latin for poison. A Roman physician by the name of Celsus had a treatment for wounds caused by rabid animals that remained widely accepted from 45 A.D. till the 1900's. Many physicians in history have described the symptoms of rabies, came up with cures and temporary ailments for the sick. The first case of rabies reported in the Americas was in Mexico by a priest in 1703. The disease swept the entire world slowly over centuries until it was noted on every continent on the globe except Antartica. [4]
In 1804 a German scientist by the name of Zinke begins conducting experiments using infected saliva on various animals, confirming that the disease does come from other infected animals. The key turning point in history for rabies comes in the 1880's when Louis Pasteur, a chemist, and his assistant, scientist, and physician, Emile Roux begin research for a cure to rabies. By 1883, the two have created was is to be the first vaccine against the disease, but the real test to prove to the public that it works comes in 1885 when a nine year old Joseph Meister is bitten by a rabid dog and brought to Pasteur who puts his career on the line by testing the new vaccine for the first time. The vaccine was a success and young Joseph Meister became the first person to be successfully treated for the disease. Pasteur being a chemist not a doctor is given immediate praise and hailed as a hero. This new vaccine is still used to this day and is still the standard treatment of rabies. The vaccine also works towards animals such as dogs leading to the campaigns of vaccinating pets to prevent the spread of disease.
Virology
Structure
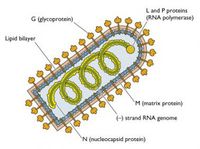
Rhabdoviruses are negative stand RNA virues, having only a single strand of RNA genome that reads the opposite of the coding strand needed to produce protiens. The virus also contains and uses its own RNA dependent RNA polymerase to make the coding strand necessary for protein production. The overall shape of the virus is 'rod shaped', with one flat end and the other rounded, giving a bullet appearance. The average diameter is roughly 100 nanometers and a length of approximately 400 nanometers, however, the actual sizes of the virus particles do vary some. The virus's envelope consists of host plasma membrane Phospholipid bi-layers. The virus contains and codes for five proteins. [5]
'G' Surface protein is a glycoprotein that forms a trimer. there excists about 1200 'G' proteins or 400 trimers per virus particle. The protein has a N-terminal signal sequence and is a transmambrane, crossing both phospholipid bi-layers. The 'G' proteins function is to bind to cell receptors on the surface of target cells, such as neurons in the central nervous system. This protein is also the target of antibodies and immunoglobulins from the adaptive immune response. There are three N-glyosidicaly attached sugar chains to the G protein. The virus enters the hosts cells cytoplasm through the endocytic pathway. When the virus becomes engulfed, the pH will drop, however, the sugar chain becomes stabilized at pH 6.1 and allows for the hydrophobic regions of the molecules to become embedded into the membrane of the hosts cell.
'M' Matrix protein is peripheral membrane protein that can be found in the inner membrane of the virus. The function of this protein not fully understood, but it is thought to act as a communicator between the 'G' protein and the nucleocapsid.
The Nucleocapsid is a striated structure inside the viral particle and takes on a helical shape. This structure consists of two proteins, the 'N' Nucleoprotein, and collectively the 'L' Large protein and the 'NS' Nonstructural or sometimes known as 'P' Phosphol protein.
'N' Nucleoprotein is a structural protein whose function is to cover the RNA for protection and allow for its transcription.
'P' Phosho Protein, having both the 'L' and 'NS', makes the RNA dependent RNA polymerase. The function of this complex is to generate the coding strand of the RNA contained in the virus perticle. This polymerase is approximately 240 kiloDalton's and roughly 60% of the viral RNA genome.
Replication
How the virus binds to a target via its receptors is not completely understood, but it is suggested through experimentation that the phospholipids, more specifically the phosphatidyl serine is involved in this process. Transcription occurs after the virus has been engulfed through endocytosis. The nucleocapsid is still contained within the virus capsule and now needs to reach cellular ribosomes in order to be transcribed. Once the pH inside the endosome drops, the membranes of the virus and endosome fuse, and the nucleocapsid is released into the cytoplasm. Multiple copies of the RNA are synthesized from the negative strand. Transcription takes place with the RNA being processed into five mRNA strand that make up some of the proteins encoded by the viral genome. Before the mRNA can be translated it is first capped, methylated, and polyadenylated. After translation of the mRNA into proteins, 'G' proteins is incorporated into the membrane of the endoplasmic reticulum and is transported to the cells surface via the golgi apparatus. Patches of these 'G' proteins then associate with 'M' proteins. A new nucleocapsid forms inside the cytoplasm from the synthesized proteins 'N','L', and 'P'. Formation of the nucleocapsid associates with the 'M' protein at the membrane budding off of the membrane with the new virus particle complete.
Pathogenisis
Transmission
Infection to humans usually occurs through a bite or scratch from an infected animal. Saliva from infected animals, as well as humans, can cause rabies when in contact with mucous tissues or recent skin wounds. Inhalation of aerosols containing virus particles as well as transplant of viral infected tissues have also been documented as possible forms of transmission. Many animals have the potential to carry the virus, but the most common source is dogs. Bats are a common source in the Americas. Other animals known to carry the disease include foxes, raccoons, skunks, jackals, and mongooses. Human to human infection through bite or scratch is hypothetically possible, but has never been confirmed.
Incubation
The rabies virus has a fairly long incubation period in comparison to many other viruses. Average time from inoculation to symptoms is approximately 1-3 months, however, cases have been reported of symptoms showing in as little as 1 week, and as long as 6 years. The incubation is dependent on where the virus infects the host and the amount of virus particles inoculating the host. The more virus particles that enter the host and closer to the central nervous system, the shorter the incubation period.
Disease
Incubation phase of the disease begins to takes place immediately after exposure. The virus binds to muscle and nerve cells at the site of the exposure through nicotinic acetylcholine receptors. Replication can occur in the muscles of the host without any signs of symptoms for a prolonged period of time. The incubation time is effected by several factors, including amount of particles exposed to and where the virus enters the host.
Prodormal Phase consists of the viruses travel from the site of infection to the central nervous system. the virus travels to the spinal cord via the axons of nerves using retrograde transportation. Once at the dorsal root ganglia and spinal cord the disease then spreads to the brain. several parts of the brain are susceptible to the virus including the cerebellum, hippocampus, Purkinje cells, and Pontine nuclei.
The Neurological phase includes the most prodominant and extreme symptoms of the disease. Once the host reaches this stage of infection, the disease becomes absolutely lethal, effectively 100% of all hosts die from the disease. Infection of the brain causes encephalitis and neural degridation, however, it is worth noting that the virus does not cause much of a cytopathic effect. The infection of the brain will lead to coma and death. The virus may also during the neurological spread to other parts of the body via the neurons including the eyes, skin, kidneys, pancrease, and adrenals.
Symptoms
During the incubation/Prodormal periods of the disease, symptoms may include itching and burning sensation at the wound or bite, fever, headache, and gastrointestinal problems. As the disease progresses into the neurological phase, about half of all cases begin to show hydrophobia and pains associated with drinking water. Other possible symptoms include hallucinations and seizures from neuron degradation in the central nervous system. The virus can lead to many various symptoms not all are required for a clinical diagnosis, but all eventually lead to respiratory failure. Some cases have been documented only showing symptoms of paralysis leading to coma and eventually respiratory failure. The symptoms of rabies can be vivid and graphic in their appearance, but none can completely confirm the diseases presence in a host.
Host Immune Response
Human testing is considered to be extremely unethical considering that the viruses extremely lethal. Testing has been conducted in animal models for immune responses and have come back with mixed results for various species of animals and the virus. An example if this is that mouse models have been used for testing with three forms of the virus including the wild type rabies (WTRV), silver-haired bat rabies virus (SHBRV), and attenuated virus. When using the wild type (WTRV), little to no immune response was detected from the mice, however, when testing with the Silver-haired (SHBRV) a inflammitory response can clearly be seen. The same inflammetory response can be seen when using an attenuated form of the wild type virus.
Diagnosis
No tests are currently available prior to symptoms to test for the viruses presence. The Virus can be confirmed via intra vitam and post mortem procedures that detect the virus as a whole, the viral antigens from host production, or the nucleic acids of the virus from infected tissues. Examples of these detection methods include reverse transcription polymerase chain reaction (RT PCR) test kits, negri bodies found in neuron cells, use of florescent tagged antibodies in infected tissues, live viral culturing (including WI-38, BHK-21, or CER), and several serological tests (including MNT, RFFIT, and EIA). Some of the tests can even be combined such as using IF on live tissue cultures to test for presence of rabies antigens.
Treatment
Rabies is an extremely lethal disease with a almost absolute 100% death rate in all hosts in the late stages of the disease. there are only a hand full of documented human cases in which a person has the late stages of the disease and survives. The only way to treat the disease is before late stages of the virus can develop in the brain by using classical vaccination and antibodies treatments. Post-Exposure Prophylaxis is the main course of action needed to prevent rabies from causing disease. The type of interaction with a suspected infected animals dictates the specific action necessary for treatment and is generally understood in three categories.
(1) Touching, Feeding, and licking on skin require no PEP.
(2) Nibbling of uncovered skin, minor scratches with no bleeding may require immediate local treatment of wound and vaccination is recommended as a precautionary measure to prevent the disease.
(3) 1 or more trans-dermal bites or scratches or lick on broken skin, as well as contact of mucous membrane with saliva will require local treatment of wound(s), vaccination, and likely use of rabies immunoglobulin to prevent and counteract to virus.
Physical and chemical methods of cleaning wounds are recommended as effective methods of removal of the virus. Some examples of these methods include Rinsing the wound with water, soap, detergent, and providone iodine.
Epidemiology
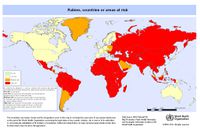
Rabies is primarily transmitted through animal bites. Many animals can be possible vectors for the virus, however, world wide most cases are caused by dogs in poor rural areas. The most common and relevant areas in which rabies is spread through dogs are Latin America, Asia, and Africa.
Between 1990 and 2004, there were 47 confirmed cases of rabies in the United States. Of those 47, 35 cases were associated with bats, and the final 12 came from dog/coyote bites. 10 of the 12 rabies cases were associated with dogs/coyotes from outside the U.S., with only 2 caused by dogs within the United States. Rabies is carried primarily through large mammals such as dogs, coyotes, raccoons, skunks, and foxes. The virus has been known to come from small rodents (woodchucks mostly), but only on rare occasions. Dogs, cats, and cattle are potential vectors for the disease in the United States. Large efforts have been put together from multiple organizations to prevent the spread of rabies from these animals using vaccinations. Bats in the U.S. have become a concern because the bats have small, very sharp teeth that may or may not be felt by people around the animals. Many bats aren't infected, but people should take caution with any contact with them. In many cases that involve rabies transmitted form bats, a fair number of people do not remember having been bitten. There have been cases of people simply handling bats that have been found in houses, barns, or in yards that became infected with the virus.
Awareness of the disease is known to most people, especially when interacting with animals acting strangely or aggresively, however, symptoms of the disease may not have developed yet or go unnoticed in animals such as bats, leading to people not seeking medical attention or vaccination after coming in contact with the virus. Educating the public to the dangers of handling or being bitten by bats as well as other animals is key in making people aware of the rabies virus and how to deal with encounters with known carries.
Prevention
Eliminating Rabies in Dogs
The disease is absolutely preventable with the use of the vaccine. [6] Do to the cost of health care, the best way to prevent the spread of rabies to humans is by eliminating rabies in animal (especially dog) populations. Vaccinating pets and controlling the wild life that comes in cantact with them are good preventative measures. Spade or neuter pets will help to decrease the number of stray animals and reporting any sick or stray animals to local animal control will also prevent the spread of rabies in near by areas. Rabies has been completely eradicated from a few countries, but there have been recent increases of the disease in Asia, Africa, and Latin America suggesting that the virus is becoming a re-emerging pathogen. By preventing rabies in animals such as dogs, the number of people needing Post-exposure prophylaxis treatment is significantly diminished.
Preventative Immunizations in People
Vaccinations can be used as pre-exposure preventative measures of immunization. People spending a lot of time in rural outdoor areas, especially travelers to high risk regions are recommended for vaccination. High risk jobs such as laboratory scientist working around the rabies virus, jobs that require working around bats, carnivores, and other mammals known to carry rabies in high risk areas are also recommended for pre-exposure vaccination. Children are at a higher risk of becoming infected because of close contact and play with animals. Children may be bitten and not tell others about bites they may have received and are recommended to receive vaccination.
References
1 NCBI. Rabies Genome. Web. 22 July 2013 <http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=11292>
2 Wikipedia. Rabies. 21 July 2013. Web. 22 July 2013 <http://en.wikipedia.org/wiki/Rabies>
3 WHO. Rabies fact sheet. July 2013. Web. 22 July 2013 <http://www.who.int/mediacentre/factsheets/fs099/en/>
4 Baer GM, The Natural History of Rabies, 2nd Edition. Elsevier Inc., 1975. Print.
5 Hunt R, Virology, Chapter 20 Rabies. The Board of Trustees of the University of South Carolina. 2011. Web. 22 July 2013 <http://pathmicro.med.sc.edu/virol/rabies.htm>
6 CDC. Rabies. 15 February 2012. Web. 22 July 2013 <http://www.cdc.gov/rabies/symptoms/index.html>

Created by Daniel Robinson, student of the University of Oklahoma, taught by Dr. Tyrrell Conway, study abraod program, summer 2013 in Arezzo, Tuscana, Italia.
