Utilization of Bacillus thuringiensis in Genetically Modified Crops
Introduction
By Zoë Frazier
The bacterium Bacillus thuringiensis is a gram-positive and rod-shaped microbe that is 2–5 µm in length and 1.0 µm wide [9]. It is found in soil ecosystems, moist environments, and within caterpillar guts [5]. Bacillus thuringiensis is non-pathogenic for most organisms expect for a variety of insect species. Bacillus thuringiensis contains a gene that is known to code for Cry proteins. These proteins are produced during the sporulation phase of the Bacillus thuringiensis lifecycle. The Cry proteins are crystalline in structure and incredibly toxin to insects if consumed. The insect species that are killed by a variety of different Bacillus thuringiensis strains include vegetable insects (tomato and tobacco hornworms), field crop insects (European corn borers, alfalfa caterpillars and webworms), fruit crop insects (Leaf roller and Achemon sphinx), and tree insects (tent caterpillars, fall webworm, pine butterfly, Western spruce budworm). Additionally, mosquitoes, black flies, fungus gnats, cottonwood leaf beetles, elm leaf beetles, and Colorado potato beetles can also be affected if they consume the toxic Cry proteins [4].
Historically the Cry proteins produced by Bacillus thuringiensis have been used as a topical pesticide. However, due to advances in biotechnology, scientists have developed a mechanism to extract the Cry gene and insert it into another organism’s genome. The Cry gene is inserted into common crops that are in high demand, such as corn, wheat, cotton, canola, soy, and potato crops. Once the Cry gene is inserted into the genome, the plant can express its own insecticidal properties. This genetic alteration results in plants that are continuously producing pesticides, which helps to cut down or even eliminate the usage of spray-on pesticides. This new technological advancement is so popular in agriculture that by 2014, a total of 93% of all corn crops in the United States contained the Cry gene [3]. There are many different strains of Bacillus thuringiensis, all of which can be used to genetically modify plants to be resistant to specific target organisms. Despite the many seeming advantages to using genetically modified crops, there are several ecological effects that could drastically change the success rate of Bt crops. Both target organisms and secondary pests have been observed to adapt or evolve to resist the toxic effects of Bt crops. The following page will further describe the integration of Bacillus thuringiensis into our modern day agricultural system along with the social and ecological implications associated with the usage of this microbe.
History
In 1901, a Japanese scientist by the name of Shigetane Ishiwata was the first to discover Bacillus thuringiensis. In the early twentieth century, Bacillus thuringiensis was the cause of a widespread disease that targeted silkworms. Ishiwata was able to isolate the microbe from samples of dead silkworm larvae. In 1927, a German scientist named Ernst Berliner isolated another strain of Bacillus thuringiensis and observed that the microbe developed endospores and parasporal crystals simultaneously. It was not until 1955 that Christopher Hannay finally determined that the parasporal crystals directly caused insect death. This discovery sparked research involving the potential uses for this naturally occurring insecticide. Finally in 1961, the first Bt-based insecticide was registered by the US Environmental Protection Agency to be used in the United States. However, the market for the Bacillus thuringiensis pesticide properties changed drastically in 1982 when the technology for the genetic modification of plants was developed. Scientists discovered that if they extracted the Cry gene from Bacillus thuringiensis, inserted it into a plasmid, and integrated that plasmid into a plants genome, the plant would express a resistance to insect activity. After several years of working with the plasmid, the first crop of transgenic plants was grown in 1996 [5].
Structure and Phylogeny
Bacillus thuringiensis is a rod-shaped microbe that appears in pairs or short chains. It is a gram-positive organism that is motile. Bacillus thuringiensis contains the hag gene, which encodes for the expression of flagellin. This protein is produced in large quantities and comes together to form the microbe’s flagellum. The presence of the hag gene is crucial for the process of H serotyping, which allows us to categorize different bacterial strains based on their antigens [9]. However, H serotyping sometimes is not able to differentiate all of the different strains of Bacillus thuringiensis. Additional technology for identifying the different Bacillus thuringiensis strains has been developed due to the importance of identifying novel strains.
Bacillus thuringiensis belongs to the phylum Firmicutes, which is known to include gram-positive bacteria that produce endospores. Bacillus thuringiensis is also in the genus Bacilli, which is characterized by rod-shaped bacteria that can be aerobic or facultative anaerobes. The closest relatives to Bacillus thuringiensis are Bacillus cereus, Bacillus anthracis, and Bacillus mycoides. Although they are all close relatives, Bacillus thuringiensis is unique due to its sporulation products and environmental habitats. During sporulation, Bacillus thuringiensis produces a crystalline protein byproduct. Additionally, Bacillus thuringiensis can live in the midgut of insects and in environments that are free of other gram-positive, spore-forming, bacillus bacteria.
Interestingly, a small number of strains of Bacillus thuringiensis are known to be pathogenic to humans. Genetic sequencing of these strains shows that the insecticidal Cry genes are not present. In fact, genetic analysis shows that the two pathogenic strains, Bt 97-27 and Bt Al Hakam, are more closely related to Bacillus anthracis and Bacillus cereus than other strains of Bacillus thuringiensis. Additional analysis of 16S and 23S rDNA nucleotide sequences show that Bacillus thuringiensis and Bacillus cereus must exchange genetic material and a fairly high frequency in their natural environment [9]. Overall, there are a total of five known Bacillus thuringiensis strains, but novel strains are still being discovered.
Life Cycle
Figure 2.
Bt Toxins

Figure 3.
Bt Crops
Figure 4.
Ethical Issues Surrounding Bt Crops
Include some current research, with at least one figure showing data.
Evolved Resistance and Secondary Pests
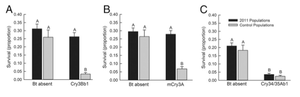
Figure 5.
Conclusion
Include conclusion
References
[1] Aeschbacher, K., Messikommer, R., Meile, L. & Wenk, C. (2005). Bt176 Corn in Poultry Nutrition: Physiological Characteristics and Fate of Recombinant Plant DNA in Chickens. Poultry Science Association, 84, 385 – 394.
[2] Catarino, R., G. Ceddia, F.J. Areal, and J. Park. (2015). The impact of secondary pests on Bacillus thuringiensis (Bt) crops. Plant Biotechnology Journal, 1-12.
[3] Cheeke, T.E., U.M. Schütte, C.M. Hemmerich, M.B. Cruzan, T.N. Rosenstiel, and J.D. Bever. (2015). Spatial soil heterogeneity has a greater effect on symbiotic aburscular mycorrhizal fungal communities and plant growth than genetic modification with Bacillus thuringiensis toxin genes. Molecular Ecology, 1-33.
[4] Cranshaw, W.S. Bacillus thuringiensis: Fact Sheet No. 5.556. Insect Series, Home and Garden. Colorado State Univserity.
[5] de Maagd, R.A. 2015. Chapter 20: Bacillus thuringiensis- Based Products for Insect Pest Control. Principles of Plant-Microbe Interactions. Springer International Switzerland, 2015. 185-192.
[6] Finucane, M.L. & J.L. Holup. (2005). Psychosocial and cultural factors affecting the perceived risk of genetically modified food: an overview of the literature. Social Science & Medicine, 60, 1603 – 1612.
[7] Gassmann, A.J. J.L., Petzold-Maxwell, E.H. Clifton, M.W. Dunbar, A.M. Hoffmann, D.A. Ingber, and R.S. Keweshan. (2014). Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. PNAS, 111(14), 5141-5146.
[8]Hansen Jesse, L.C. & J.J. Obrlycki. (2000). Field deposition of Bt transgenic corn pollen: lethal effects on the monarch butterfly. Oecologia, 125, 241 – 248.
[9] Ibrahim, M.A., N. Griko, M. Junker, and L.A. Bulla. 2010. Bacillus thuringiensis: A genomic and proteomics perspective. Bioengineered Bugs 1:1, 31-50.
[10] Jurat-Fuentes, J.L. "What Is Bacillus Thuringiensis (Bt)?" Bt Mode of Action. The University of Tennessee Institute of Agriculture. Web. <http://web.utk.edu/~jurat/Btresearchtable.html>.
[11] Petras, S.F. and L.E. Casida. (1985). Survival of Bacillus thuringiensis Spores in Soil. Applied and Environmental Microbiology, 59, 1496 – 1501.
[12] Uzogara, S.G. (2000). The impact of genetic modification of human foods in the 21st century: A review. Biotechnology Advances, 18, 179 – 206.

