Celiac Disease
By Kay Burrows
Introduction
Celiac disease (coeliac disease, celiac sprue) is caused by an autoimmune disorder in which the affected person experiences an attack on intestinal villi following the ingestion of gluten, a protein found in wheat [4]. Gluten is comprised of two separate classes of proteins called gliaden and glutenin. Similarly, the protein agonists in barley and rye, hordeins and secalins, can also trigger the immune response characterized by Celiac disease. These proteins are resistant to being broken down by gastric acid, the digestive element used in what is commonly known as stomach acid, because they contain higher levels of glutamines and prolines. The proteins are also resistant to digestion from enzymes in the pancreas. Oats, however, containing of a protein called avenin, are rarely responsible for inflammation [5].
When a patient experiences the autoimmune attack associated with these proteins, the intestinal function is greatly reduced, often leading to malabsorption, anemia, and in untreated cases, effects as serious as infertility, multiple sclerosis, and various neurological and psychological deficits. Those affected often also suffer from other autoimmune diseases including type 1 diabetes, alopecia, and autoimmune hepatitis [1].
Celiac disease currently affects an estimated 1% of the total world population; however, due to its wide range in severity and general lack of public knowledge about the disease, as many as 97% of those affected with the disease in the United States alone are undiagnosed [6].
The disease can be triggered at any age, and the likelihood for a first-degree relative to also have celiac disease stands between 8 and 18% [6]. While there are certainly genetic predispositions for a person to develop the disease, it is often triggered by a combination of known and unknown factors including the onset of other diseases and environmental factors such a diet and physical stress.
The methods of the disease’s destruction of the intestinal villi, as well as the effects of that destruction on the absorption of basic nutrients are vaguely understood in the medical community, with some debate over the precise mechanisms by which Celiac causes autoimmunity. However, the connections between Celiac disease and other detrimental digestive issues is a topic that is under even heavier examination in modern research. With increasing interest in the way human gut microbiota affect digestion and absorption, researchers are beginning to examine the state of microbiota diversity that may affect the trigger of Celiac disease as well as the resulting diversity post-diagnosis. It is possible that these fluctuating beneficial bacteria, if unable to aid with the digestion of gluten proteins, may influence the environment necessary to spur an immune response to the presence of the proteins in the gut of a Celiac patient.
Symptoms
It is estimated that approximately 3 million Americans are affected by Celiac disease, but that up to 97% of that 3 million are undiagnosed [6]. The cause for such low reports of Celiac disease compared to predicted values for Celiac prevalence can likely be attributed to misinformation about the spectrum of severity of the disease symptoms as well as misdiagnosis or misunderstanding of typical gastrointestinal issues. A Celiac disease patient can experience several seemingly unrelated symptoms, and may not experience the classic array of symptoms associated with gastrointestinal stress. Symptoms also vary in severity, and often overlap with other symptoms of related and simultaneously occurring illnesses associated with Celiac [1, 2].
Commonly, a patient can come in to their family physician with complaints about fatigue or lightheadedness, which can be a result of a number of physical ailments. Unless paired with a gastrointestinal complaint, these issues are often misdiagnosed for stress, sleep deprivation, or dietary issues. If the patient without digestive complaints goes as far as to receive blood tests, they will find two very ambiguous signs that are actually rather typical of serious Celiac cases: low ferritin stores in the blood, hand in hand with a diagnosis of anemia [3]. Without further investigation towards cause, the patient may be advised to take supplemental iron or encouraged to consume more red meats or leafy greens in hopes of restoring normal iron levels and therefore reversing iron-deficiency induced anemia.
A more severe untreated case of the disease can produce an unusual combination of symptoms. Often, a “typical” Celiac patient will report diarrhea, constant or frequent bloating after eating, nausea, and/or discolored stools (frequently yellow or dark gold)[1]. Patients may report these symptoms regardless of very recent gluten intake due to the constant, slow-healing inflammation caused by the immune response to gluten in the past. However, even the most classic symptom, diarrhea, occurs in less than half of diagnosed cases of the disease [4]. Other unusual symptoms include red rashes-- often dry and itchy, appearing on various body parts including the abdomen, chest, and/or arms, difficulty focusing on work, chronic fatigue, vomiting, growth issues in children, and osteoporosis in adults. Younger children often show more obvious signs of Celiac disease, including and not limited to diarrhea and malabsorption, while older children tend to present symptoms such as abdominal pain or constipation. Adults are more likely to experience serious but seemingly unrelated symptoms resulting from long-term manifestation of the disease including anemia, irritable bowel syndrome, and reduced bone density [2].
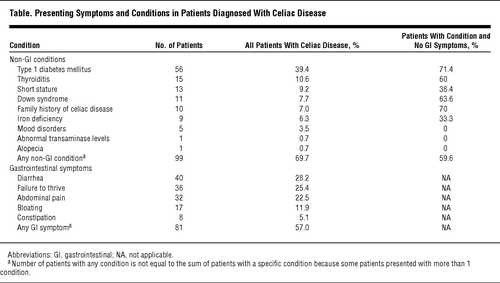
A recent study from the Medical College of Wisconsin evaluated other connections to Celiac disease and discussed whether or not these would better serve as predictive diagnostic tools (see table, right). With many patients not experiencing gastrointestinal symptoms, physicians can consider the prevalence of Celiac disease in other disorders and diseases, such as Type 1 diabetes, Down syndrome, and Thyroiditis, as a predictive tool in the diagnosis of the disease [2]. Early diagnosis of Celiac disease by testing high-risk groups such as these could help prevent further intestinal damage and prevent the onset of various complications associated with untreated Celiac disease, especially in younger children who may not be able to vocalize their symptoms. The study also highlights a growing problem for detecting Celiac as a possible explanation for various physical ailments: the percentage of those with the disease that present classic gastrointestinal symptoms is actually decreasing over time. Physicians are becoming more aware that the disease can present itself in usual ways, and must react accordingly to catch these problems before they progress.
Diagnosis
Diagnosis of Celiac disease can be a multi-step procedure, or a simple recommendation. Patients experiencing symptoms of Celiac disease may first simply be advised to reduce or eliminate their intake of foods containing gluten protein (wheat, rye, barley) in their diet. If they notice a difference in gastrointestinal health—less bloating, firmer stools, better digestion—they may choose not to continue testing for Celiac disease, and rather decide to eliminate gluten altogether and accept gluten sensitivity as enough evidence of the disease [3].
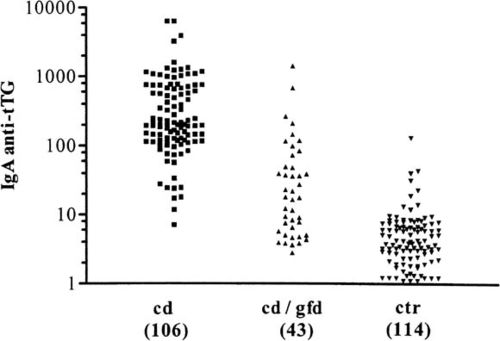
In more unclear cases or in situations where patients choose not to try a gluten-free diet, an antibody test can determine the presence of Celiac disease. Because Celiac is an autoimmune disease, tests for certain antibodies can determine if the intestine is currently under a gluten-induced attack from the immune system. The primary antibody investigated in these simple blood tests is immunoglobulin A (IgA) anti-tissue transglutaminase antibody. An elevated level of this antibody is a very reliable indication of Celiac inflammation; this is widely considered the most sensitive and specific Celiac predictor, but it doesn’t come without faults. A quantitative immunoglobulin A test will be taken simultaneously to rule out the possibility of a false negative caused by a deficiency in IgA which occurs in about 3% of positive celiac cases [3].
A study conducted in 1997 (Dieterich et. al) elaborates on the efficacy of testing for IgA, examining whether or not anti-tTG is the major autoantigen produced from an autoimmune response following the ingestion of gluten or gluten-like proteins. They found that anti-tTG is in fact the major, if not the only autoantigen produced in these reactions, further clarifying the importance of the IgA test in identifying Celiac-caused autoimmune response and inflammation [7].
A test for deamidated gliadin peptide antibodies may be taken for children under two or under the case that the anti-tTG test comes back negative in order to further investigate whether a patient should be monitored for the possibility of Celiac disease. Less frequent antibody tests include a test for anti-endomysial antibodies, which are antibodies that indicate ongoing intestinal damage, and tests for anti-reticulin antibodies that are found in just over half of those later diagnosed with Celiac disease [3].
To determine the severity of the disease and any further complications, a physician may order several other blood tests to measure deficiencies. Such tests include a complete blood count test to investigate possible anemia, erythrocyte sedimentation & c-reactive protein tests to determine the presence of intestinal inflammation, tests to determine deficiencies in vitamin D, B12, and iron, and a stool fat test to determine the extent of malabsorption resulting from the inflammation of the intestinal wall. A test for Anti-F-actin that comes back positive may give health care providers information about the damage inflicted upon the intestinal wall. Physicians may also choose to investigate other sources of inflammation such as food allergies or lactose intolerance that are more likely to occur in conjunction with Celiac disease [3].
Patients may suspect they are at risk for developing Celiac disease due to a family history of the disease or a commonly associated illness or disorder. If they do not feel symptomatic at the time, they may request a genetic test to determine genetic influence for Celiac disease. Unfortunately, these tests are currently very expensive and cannot determine a certainty for an individual to later develop the disease. Because of this, most individuals wait until they feel symptomatic in order to request tests for the disease[1,3].
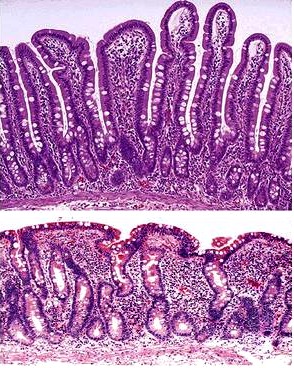
When any of these diagnostic tests support the presence of Celiac disease, the next step for a patient is a biopsy of the wall of the small intestine, confirming the diagnosis of the disease. This invasive procedure involves removing several small pieces of tissue from the wall of the small intestine and examining the sampled tissue under a microscope[1]. For a patient with Celiac disease, physicians would expect to see two major differences between the intestinal wall affected by Celiac disease and a normal intestinal wall: the presence of short, blunted intestinal villi as opposed to long, finger-like villi, called villous atrophy, and an increased crypt length between villi, called crypt hyperplasia[8].
With several pieces of evidence, diagnosing celiac disease is an uncomplicated process. The complexity associated with Celiac diagnosis comes from a misunderstanding of the variation in symptoms and the misdiagnosis due to a lack of investigation for Celiac disease.
Disease Pathophysiology
Genetic influence for a Celiac patient’s flaw in immune response is located in the class of genes regarding the human leukocyte antigen, HLA-DQ2 and HLA-DQ2. These HLA genes, located on chromosome 6 in three distinct classes, are responsible for the mechanism by which anitgens are presented to immune cells. In these class II genes, the main responsibility is the outer presentation of peptides. With the correct combination of these gene types, the immune systems of Celiac patients are prone to mistakes in recognizing antigens that are foreign to the body (in this case, gliadin and other proteins) as opposed to those produced by the body (such as the tTG2 enzyme)—in essence, the cause of autoimmunity. This lack of distinction can clarify why patients with Celiac often are victims of other autoimmune diseases and problems [5].
When an individual affected by Celiac disease consumes wheat, barley, or rye, the undigested gliadin, glutenin, hordein or secalin dissociate from the substance and move to the lamina propria, the layer of connective tissue located just under the epithelial cells that make up the intestinal villi. There, tissue transglutaminase-2 enzyme (tTG2) changes the proteins’ glutamine to negatively-charged glutamate. The complex created by the connection between these proteins and tTG2 is the proposed starting point at which Celiac disease becomes an autoimmune disease [5].
The proteins trigger the response of the body’s immune system, in which the T cells initially target the gliadin protein and then end up targeting also the protein-enzyme complex by mistake. This ultimately causes the B cells to produce an autoantibody towards tTG2, alongside the cytotoxic response to gliadin itself. These attacks are the root of the autoimmune response that causes intestinal inflammation in those affected by Celiac disease [5].
When the villi are subsequently attacked by the patient’s immune system, they become blunt and enflamed, unable to move freely in their environment. The intestinal wall become chronically enflamed so long as the patient continues to consume gluten and gluten-like proteins, as the proteins will perpetuate the body’s autoimmune response and therefore perpetuate the attack on intestinal villi. Chronic inflammation of the villi in the intestine causes the villi to be unable to trap and absorb nutrients from food, leaving the individual deficient of many essential vitamins and minerals. Where a healthy patient’s intestine has long, fingerlike villi capable of moving particles along the surface of the intestinal wall and absorbing nutrients due to the textured nature of the intestine, the patient with Celiac disease has an intestinal wall that appears smooth and uniform, unable to stick to or trap particles. This chronic inflammation is the basis of Celiac disease and all of its subsequent issues—diarrhea from the inability to properly digest particles and anemia from the inability to absorb vitamins and minerals necessary to keep the blood in good shape [5,1].
_
Effects on Intestinal Microbiota
Recent research has addressed the effects of Celiac disease on the diversity of the naturally occurring microbiota in the intestine. A healthy individual has a diverse combination of bacterial species occupying the intestine, and there is an ongoing interest in the medical community for the purpose and function of these bacteria for the human body. While widely regarded as beneficial for gut function and absorption of nutrients, many of these bacterial colonies are uncharacterized at the time being. What is known, however, is that an imbalance in these delicate communities can greatly influence human health and gut function.
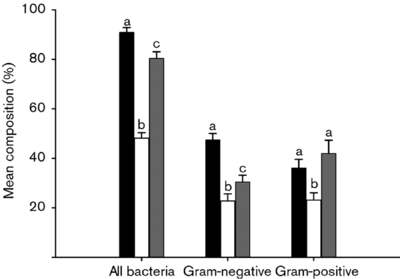
A change in the intestinal environment of these bacteria ultimately could have the potential to influence the different colonies of gut microbiota. One study (Nistal et Al, 2012) showed that the microbiota occupying healthy adults, adults with Celiac disease undergoing treatment, and untreated adult individuals with Celiac disease had distinctly unique bacterial colonies in the small intestine. Furthermore, they found that these microbiota were also different from the microbiota of children in the same three categories [9].
A similar study (Nadal I et al, 2007) found that that children affected by Celiac disease had intestinal microbiota with a “higher incidence of Gram-negative and potentially pro-inflammatory bacteria.” Apart from these differences, certain bacteria including Atopobim, Clostridium histolyticum, and Faecalibacterium prausnitzii, remained relatively constant in both Celiac individuals and non-Celiac individuals [10].
While current research efforts have uncovered more about the microbial effects of Celiac patient's dysfunctional immunity in the gut, we have yet to uncover the true meaning behind these studies. A question of which contributes to which is at hand when considering the differences in microbiota communities in the small intestine of those who are ultimately diagnosed with Celiac disease and those who are unaffected by the disease.
-
Current Treatments & Long Term Effects
Current treatments for Celiac disease are simple and straightforward. Recently diagnosed Celiac patients are advised to immediately and permanently cut all foods from their diet that would contain even trace amount of gluten, mainly including wheat, barley, and rye [1] For most, this means cutting out pasta, bread of any kind, many desserts and sauces with trace amounts of flour, beer, and other more complicated dishes included any kind of breaded meats and French fries or other foods fried in the same oil as gluten-containing foods.
Those with Celiac disease can find alternative recipes containing rice, soy, coconut, or other products that help produce some of the same goods that wheat would—rice flour and rice noodles are popular alternative for these difficult transitions. As awareness increases for the implications of the presence of gluten in foods, many manufacturers are careful to label even products made in the same facilities as those made with gluten. With this increase in awareness also comes a demand for a more diverse supply of alternative foods, hopefully one day providing celiac patients with a greater number of options for alternative foods.
In a small number of cases, untreated Celiac disease can lead to intestinal damage so severe that it cannot be reversed on a gluten-free diet. The villi in these cases have become so damaged from autoimmune attacks that they can no longer function to trap nutrients. In these cases, patients may need to be provided nutrient intravenously to supplement for the nutrients they can no longer absorb through their damaged intestinal wall.
Long term untreated Celiac disease can have life threatening consequences. In young children, undetected Celiac disease can lead to failure to thrive, and ultimately death. In adults, the disease can lead to severe depression or anxiety, epileptic seizures, cancer, and infertility [4]. While some patients may be able to survive several years without knowing they are suffering from the disease, ultimately patients are struck with malabsorption and are forced to investigate the cause or suffer indefinitely.
Further research may seek to make celiac testing more accessible and more affordable in order to increase the probability of diagnosing the disease and prevent further intestinal damage. Research may also become pointed towards possible links between Celiac and other autoimmune disorders with temporary and permanent psychological disorders, as well as determine the long term effects of microbiota changes caused by the disease [5].
References
[1] https://my.clevelandclinic.org/health/diseases_conditions/hic_celiac_disease
[2] http://archpedi.jamanetwork.com/article.aspx?articleid=379068
[3] https://labtestsonline.org/understanding/analytes/celiac-disease/tab/test/
[4] https://celiac.org/celiac-disease/what-is-celiac-disease/
[5] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3872820/
[6] http://www.uchospitals.edu/pdf/uch_007937.pdf
[7] Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, and Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 3: 797-801 [8] De Sousa Moraes LF, Grzeskowiak LM, de Sales Teixeira TF, Gouveia Peluzio MdC. 2014 Intestinal microbiota and probiotics in celiac disease. Clin. Microbiol. Rev. 27, 482–489.
[9] Nistal, E., Caminero, A., Herrán, A. R., Arias, L., Vivas, S., de Morales, J. M. R., Calleja, S., de Miera, L. E. S., Arroyo, P. and Casqueiro, J. (2012), Differences of small intestinal bacteria populations in adults and children with/without celiac disease: Effect of age, gluten diet, and disease. Inflamm Bowel Dis, 18: 649–656. doi: 10.1002/ibd.21830
[10] Nadal I, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol 2007; 56: 1669–74.
Authored for BIOL 291.00 Health Service and Biomedical Analysis, taught by Joan Slonczewski, 2016, Kenyon College.