Dengue Disease

[[4]]
Etiology/Bacteriology
Taxonomy
Group = Group IV positive-sense ssRNA virus | Order = Unassigned | Family = Flaviviridae | Genus = Flavivirus | species = Dengue Virus
|
NCBI Taxonomy: (DENV-1) (DENV-2) (DENV-3) (DENV-4) Genome: Dengue Virus |
Description
Dengue (pronounced DENgee) viruses belong to the family Flaviviridae, genus Flavivirus. There are four serotypes: DENV-1, DENV-2, DENV-3, and DENV-4, which belong to a larger, heterogeneous group of viruses called arboviruses. This is an ecological classification, one which implies that transmission between vertebrate hosts, humans, is dependent on hematophagous arthropod vectors. Dengue fever is transmitted by the bite of an Aedes mosquito infected with a dengue virus. The mosquito becomes infected when it bites a person with dengue virus in their blood. It can’t be spread directly from one person to another person. Dengue fever is a painful, debilitating mosquito-borne disease. This disease has symptoms similar to the flu, however the symptoms may progress to massive bleeding, shock, and death. This is called dengue shock syndrome (DSS).This virus is related to the viruses that cause West Nile infection and yellow fever. Each year, an estimated 100 million cases of dengue fever occur worldwide, with most of these cases occurring in tropical areas of the world. [1]
The Dengue Virus is considered an Old Disease, which goes back to the Chin Dynasty (AD 265–420), where it was first recorded. There are reports of epidemics of dengue-like illnesses in the French West Indies in 1635 and in Panama in 1699. By the late 1700s, the disease had a worldwide distribution in the tropics: Indonesia, Egypt, and in 1780 in Philadelphia. [6] From the late 1700s to World War II, repeated epidemics of dengue illness occurred in most tropical and subtropical regions of the world.
Pathogenesis
Transmission
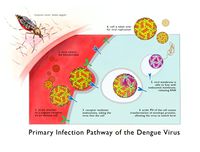
Dengue virus infections come from the bite of an infected Aedes mosquito. These mosquitoes usually live between the latitudes of 35 degrees North and 35 degrees South below an elevation of 1,000 metres (3,300 ft).[5] Mosquitoes become infected when they bite infected humans, and later transmit infection to other people they bite. The two main species of mosquito, Aedes aegypti and Aedes albopictus, have been responsible for 95% of cases of dengue transmitted, however other mosquito species such as A. albopictus, A polynesiensis and several A. scutellaris may also transmit the disease.[6] Humans are the primary host of the virus, but it may also circulate in nonhuman primates.[3] An infection may be acquired via a single bite.[4]
After taking a blood meal from an infected person, the virus infects the cells lining the mosquito's gut. About 8–10 days later, the virus spreads to other tissues including the mosquito's salivary glands and is subsequently released into its saliva. The virus has no effect on the mosquito, which remains infected for life. Dengue may also be transmitted via infected blood products and through organ donation. [5] Vertical transmission from mosquito to mosquito has also been observed in some vector species. [10]
Infectious dose, incubation, and colonization
Dengue has an incubation period of about 3–10 days, and follows a course of 3 phases: febrile, critical, and convalescent. [7] The infectious dose of DENV is unsure as of 2015. Research is still ongoing about how many viruses is required to cause an infection in the host. Difficulty may be due to the lack of a successful animal model. Growth of DENV occurs in Langerhans cells, macrophages, and later in endothelial cells of the human body. [13]
Epidemiology
With the proper healthcare, patients with the Dengue diagnosis recover. The mortality rate is 1–5% without treatment. Severe disease carries a mortality of 26%.[3] Dengue is believed to infect 50 to 100 million people a year worldwide, with hundreds of thousands of life-threatening infections requiring hospitalization resulting in approximately 12,500-25,000 deaths.[7]
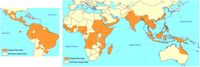
The burden of disease from dengue is estimated to be similar to other childhood and tropical diseases, such as tuberculosis, at 1600 disability-adjusted life years per million population. It is the most common viral disease transmitted by arthropods.[3] As a tropical disease it is deemed only second in importance to malaria. While once exclusively a tropical disease it has become global, and is endemic in more than 110 countries. The World Health Organization counts dengue as one of sixteen neglected tropical diseases.[7]
The incidence of dengue increased 30 fold between 1960 and 2010. This increase is believed to be due to a combination of urbanization, population growth, increased international travel, and global warming. The geographical distribution is around the equator with 70% of the total 2.5 billion people living in endemic areas from Asia and the Pacific. In the United States, the rate of dengue infection among those who return from an endemic area with a fever is 2.9–8.0%,[7] and it is the second most common infection after malaria to be diagnosed in this group.[7]
Virulence factors
The dengue virus genome is about 11000 bases that codes for structural proteins and nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, NS5), with short non-coding regions on both the 5' and 3' ends.
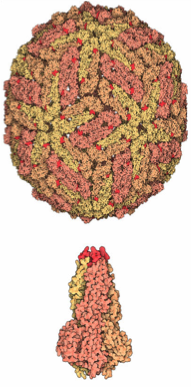
E protein: The DENV E (envelope) protein, found on the viral surface, is important in the attachment of the virus to the host cell. Several molecules which interact with the viral E protein (ICAM3-grabbing non-integrin, CD209, Rab 5,GRP 78, and the mannose receptor) have been shown to be important factors mediating attachment and viral entry. [11]
prM/M protein: The DENV prM (membrane) protein, which is important in the formation and maturation of the viral particle, consists of seven antiparallel β-strands stabilized by three disulfide bonds.[11] The glycoprotein shell of the mature DENV virion consists of 180 copies each of the E protein and M protein. The immature virion starts out with the E and prM proteins forming 90 heterodimers that give a spiky exterior to the viral particle. This immature viral particle buds into the endoplasmic reticulum and eventually travels via the secretory pathway to the Golgi apparatus. As the virion passes through the trans-Golgi Network (TGN) it is exposed to low pH. This acidic environment causes a conformational change in the E protein which disassociates it from the prM protein and causes it to form E homodimers. During this maturation, pr peptide is cleaved from the M peptide by the host protease. The M protein then acts as a transmembrane protein under the E-protein shell of the mature virion. The pr peptide stays associated with the E protein until the viral particle is released into the extracellular environment. This pr peptide covers the hydrophobic fusion loop of the E protein until the viral particle has exited the cell. [11]
NS3 protein: The DENV NS3 is a serine protease, as well as an RNA helicase and RTPase/NTPase. The protease domain consists of six β-strands arranged into two β-barrels formed by residues 1–180 of the protein. The catalytic triad (His-51, Asp-75 and Ser-135), is found between these two β-barrels, and its activity is dependent on the presence of the NS2B cofactor. [12] This cofactor wraps around the NS3 protease domain and becomes part of the active site. The remaining NS3 residues (180–618), form the three subdomains of the DENV helicase. A six-stranded parallel β-sheet surrounded by four α-helices that make up subdomains I and II. Subdomain III is composed of 4 α-helices surrounded by three shorter α-helices and two antiparallel β-strands. [11]
NS5 protein: The DENV NS5 protein is a 900 residue peptide with a methyltransferase domain at its N-terminal end (residues 1–296) and a RNA-dependent RNA polymerase (RdRp) at its C-terminal end (residues 320–900). The methyltransferase domain consists of an α/β/β sandwich flanked by N-and C-terminal subdomains. [12]
Antibody-dependent enhancement and Dengue fever: The reason that some people suffer from more severe forms of dengue, such as dengue hemorrhagic fever, is multifactorial. Different strains of viruses interacting with people with different immune backgrounds lead to a complex interaction. Among the possible causes are cross-serotypic immune response, through a mechanism known as antibody-dependent enhancement, which happens when a person who has been previously infected with dengue gets infected for the second, third or fourth time. The previous antibodies to the old strain of dengue virus now interfere with the immune response to the current strain, leading paradoxically to more virus entry and uptake. [12]
Clinical features
Symptoms
Symptoms usually begin four to six days after infection and last for up to 10 days, may include
• Sudden high fever
• Severe headaches
• Pain behind the eyes
• Severe joint and muscle pain
• Nausea
• Vomiting
Skin rashes are not uncommon, which appears three to four days after the onset of a fever. Mild bleeding (such a nose bleed, bleeding gums, or easy bruising) will occur. Sometimes symptoms are mild and can be mistaken for those of the flu or another viral infection. Younger children and people who have never had the infection before tend to have milder cases than older children and adults. Dengue hemorrhagic fever is a rare complication characterized by high fever, damage to lymph and blood vessels, bleeding from the nose and gums, enlargement of the liver, and failure of the circulatory system, which occurs during the critical stage of the disease. The symptoms may progress to massive bleeding, shock, and death, known as dengue shock syndrome (DSS). People with weakened immune systems as well as those with a second or subsequent dengue infection are at greater risk for developing dengue hemorrhagic fever. [1]
Diagnosis
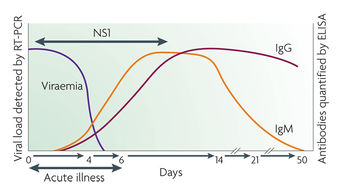
Diagnosis of dengue Fever is difficult because symptoms are similar to the flu. Usually, diagnosis is obtained by a review of symptoms and knowledge of exposure to mosquitos. Serological tests are also used to obtain a diagnosis. They look for IgM or a rise in titer of IgG in the body during infection. Finally, some immunohistochemistry infections can be diagnosed by finding antigens.
These testing Algorithms for Dengue include PCR, MAC ELISA, IgG ELISA, and PRNT. PCR of the DENV can be detected in the blood (serum) from patients for approximately the first 5 days of symptoms. A MAC ELISA IgM antibody capture ELISA (MAC-ELISA) format is most commonly used in diagnostic laboratories and commercial available diagnostic kits. The assay is based on capturing human IgM antibodies on a microtiter plate using anti-human-IgM antibody followed by the addition of dengue virus specific antigen (DENV1-4). Another test, IgG ELISA, is used for the detection of a past dengue infection utilizes the same viral antigens as the MAC ELISA. Finally, a Plaque Reduction and Neutralization Test (PRNT) and the microneutralization PRNT can be used when a serological specific diagnostic is required. This assay is the most specific serological tool for the determination of dengue antibodies. The PRNT test is used to determine the infecting serotype in convalescent sera. This assay measures the titer of the neutralizing antibodies in the serum of the infected individual and determines the level of protective antibodies this individual has towards the infecting virus.[7]
Treatment
For mild infections of Dengue Fever, there is no particular treatments. Symptoms typically clear up within seven to 7 days from the day of infection. To relieve pain from muscle sores that develop, an oral anesthetic can be used. The treatment of the general pain associated with the disease can be treated with over the counter drugs such as ibuprofen and acetaminophen. If symptoms worsen, hospitalization may be necessary.
Prevention
Prevention depends on control of and protection from the bites of the mosquito that transmits it. The World Health Organization recommends a Integrated Vector Control program consisting of five elements: (1) Advocacy, social mobilization and legislation to ensure that public health bodies and communities are strengthened, (2) collaboration between the health and other sectors (public and private), (3) an integrated approach to disease control to maximize use of resources, (4) evidence-based decision making to ensure any interventions are targeted appropriately and (5) capacity-building to ensure an adequate response to the local situation.[9]
The primary method of controlling A. aegypti is by eliminating its habitats.[9] This may be done by emptying containers of water or by adding insecticides or biological control agents to these areas. Reducing open collections of water through environmental modification is the preferred method of control, given the concerns of negative health effect from insecticides and greater logistical difficulties with control agents. People may prevent mosquito bites by wearing clothing that fully covers the skin and/or the application of insect repellent (DEET being the most effective).[9] A number of novel methods have been used to reduce mosquito numbers with some success including the placement of the fish Poecilia reticulata or copepods in standing water to eat the mosquito larva.
There are currently no approved vaccines for the dengue virus, however there are ongoing programs working on a dengue vaccine to cover all four serotypes. One of the concerns is that a vaccine may increase the risk of severe disease through antibody-dependent enhancement. [8]
Host Immune Response

The virus has an incubation period of 4-10 days after the bite from an infected mosquito. Symptoms usually last for 2-7 days post incubation. The next 24-48 hours of the critical stage can be lethal; proper medical care is needed to avoid complications and risk of death. The warning signs to look out for occur 3-7 days after the first symptoms. [2]
The virus targets Langerhans cells, which once are infected with the dengue virus, they travel from the infection site in the skin to the lymph nodes. The infected Langerhans cells display dengue viral antigens on their surface, which activates the innate immune response: macrophages. When infected with Dengue virus, the immune system produces cross-reactive antibodies that provide immunity to that particular serotype. However, these antibodies are incapable of neutralizing any other serotypes upon reinfection and actually serve to increases viral infection. When macrophages consume the ‘neutralized’ virus, the virus is able replicate within the macrophage. As the infected macrophages travel through the lymphatic system, the dengue virus spreads throughout the body. During its journey, the dengue virus infects more cells, including those in the lymph nodes and bone marrow, which induces the dengue hemorrhagic fever. [13]
References
Created by Brandon Kitchens, student of Tyrrell Conway at the University of Oklahoma.
1 Karriem-Norwood, Varnada, MD. "Dengue Fever: Symptoms, Causes, and Treatments." WebMD. WebMD, 20 Sept. 2012. Web. 24 July 2015
2Martina, B. E. E., Koraka, P., & Osterhaus, A. D. M. E. Dengue virus pathogenesis: An integrated view. Clinical Microbiology Reviews 22, 564–581 (2009). doi:10.1128/CMR.00035-09
3"Vector-Borne Viral Infections". World Health Organization. Retrieved 17 January 2011.
4 Retrieved 2010-12-23Center for Disease Control and Prevention. "Chapter 5 – Dengue Fever (DF) and Dengue Hemorrhagic Fever (DHF)". 2010 Yellow Book.
5 Wilder-Smith A, Chen LH, Massad E, Wilson ME (January 2009). "Threat of dengue to blood safety in dengue-endemic countries". Emerging Infect. Dis. 15 (1): 8–11. doi:10.3201/eid1501.071097. PMID 19116042. PMC 2660677.
6 S. B. Halstead (2007) Dengue. Lancet 370, 1644-1652
7 CDC. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 09 June 2014. Web. 24 July 2015.
8 Vaccine Development, Dengue Vaccine Initiative, November 2012, accessed November 5, 2013
9 WHO media centre (March 2009). "Dengue and dengue haemorrhagic fever". World Health Organization.. Retrieved 2010-12-27.
10Haddow, AD; Guzman, H; Popov, VL; Wood, TG; Widen, SG; Haddow, AD; Tesh, RB; Weaver, SC (Jun 5, 2013). "First isolation of Aedes flavivirus in the Western Hemisphere and evidence of vertical transmission in the mosquito Aedes (Stegomyia) albopictus (Diptera: Culicidae).". Virology 440 (2): 134–9.
11Perera R, Kuhn RJ (August 2008). "Structural proteomics of dengue virus". Current Opinion in Microbiology 11 (4): 369–77. doi:10.1016/j.mib.2008.06.004. PMC 2581888. PMID 18644250.
12Dejnirattisai W, Jumnainsong A, Onsirisakul N, et al. (May 2010). "Cross-reacting antibodies enhance dengue virus infection in humans". Science 328 (5979): 745–8. Bibcode:2010Sci...328..745D. doi:10.1126/science.1185181. PMID 20448183.
13Diamond, M. S. Evasion of innate and adaptive immunity by flaviviruses. Immunology and Cell Biology 81, 196–206 (2003). doi:10.1046/j.1440-1711.2003.01157.x