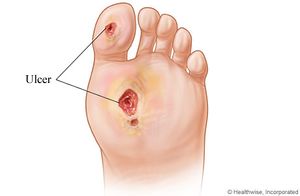
Microbial Communities in Diabetic Foot Ulcers
Introduction
Diabetes is rapidly becoming a prevalent disease in the United States. According to the Centers for Disease Control and Prevention, there are currently 21.0 million people in the United States who have been diagnosed with diabetes [13]. Of these 21.0 million people, approximately 15 percent will develop an ulcer in an extremity, generally the foot, due to diabetes. These diabetic foot ulcers are considered to be “the most common, disabling, and costly complication of diabetes” [5]. These ulcers can lead to amputations, about 80,000 per year in the United States, if the infection is prolonged and not effectively treated.
These infections are particularly tenacious because those affected with diabetes have accompanying inflammation, poor blood flow, and elevated levels of glucose, which lead to decreased rates of healing [12]. Diabetic foot ulcers are also commonly associated with peripheral arterial disease, peripheral neuropathy, or both [5]. These conditions act to increase the likelihood of developing a foot ulcer, as they can work to reduce feeling in the lower extremities, making it more likely to develop a wound that can turn into an ulcer when unchecked. Diabetic foot ulcers are also considered to be chronic wounds, so they are said to, “like all chronic wound types, heal slowly and in an unpredictable manner” [1].
Physical environment
Diabetic foot ulcers often form after some sort of trauma due to a lack of sensation from peripheral neuropathy. The trauma can then often go unnoticed, which can allow an ulcer to form. Once the skin is broken, the subcutaneous tissue provides a “moist, warm, and nutritious environment that is conducive to microbial colonization and proliferation” [1].
The microbial communities of diabetic foot ulcers often are involved in biofilm formation [3]. Biofilms occur when bacteria attach to a surface or to other bacterial members of the community already present on a surface. This creates a community that is more resilient to both the immune system and antibiotic use [10]. The cells in a biofilm are attached, unlike free-floating cells, and the tightly packed community of a biofilm acts to protect those cells that are not on the surface of the biofilm. The biofilm is also associated with increased inflammation when the infection has been present for an extended period of time. This leads to lytic enzymes and free radicals being present in the ulcer, which also exacerbates the slow healing process [6].
As an environment, diabetic foot ulcers contain areas that are aerobic and anaerobic. The section of the ulcer that is near the surface has access to oxygen, while an anaerobic environment is found deeper into the ulcerated tissue. The presence or absence of oxygen dictates which type of organisms can live in the different zones of the ulcer.
Microbial communities
The microbial communities that comprise diabetic foot ulcers are diverse, including both aerobic and anaerobic bacteria, as well as fungi, so they are considered polymicrobial wounds. Approximately 66% of these infections are polymicrobial, which can complicate treating these infections [12]. Obligate or facultative anaerobes comprise the majority of bacteria that are found in diabetic wounds [7]. 16S ribosomal RNA amplicon sequencing found that common anaerobes in diabetic foot ulcers include Staphylococcus, Corynebacterium, Peptoniphilus, Anaerococcus, Finegoldia, Porphyromonas, and Actinomyces [7]. 16S ribosomal RNA amplicon sequencing found that only between 1-5% of the operational taxonomic units were aerobes.
Microbial processes
Aerobic bacteria require oxygen to survive successfully and efficiently, so they generally live near the surface of the wound. Anaerobic bacteria do not require oxygen, and in the case of obligate anaerobes, require the absence of oxygen. This allows anaerobic bacteria to colonize the deeper areas of the diabetic foot ulcer that do not receive oxygen [12]. It has also been hypothesized that it is possible for anaerobic bacteria to survive near the surface because of the presence of biofilms. These biofilms allow the obligate anaerobes to be shielded by aerobes and facultative anaerobes, which can tolerate oxygen [7].
Most of the bacteria present in diabetic foot ulcers undergo fermentative metabolism. Fermentation is a type of heterotrophic metabolism. It uses organic molecules as a carbon and an energy source, and then utilizes pyruvate or its derivatives as an electron acceptor, resulting in the generation of fermentation products like alcohols or organic acids. Fermentation does not provide as much energy for the cell as aerobic respiration, but it is sufficient to provide energy for a cell [4]. An example of this process is seen in Staphylococcus, a common bacterial member of diabetic foot ulcers. Staphylococcus can utilize either aerobic respiration or fermentation, but fermentation is more common in diabetic foot ulcers [9]. The process of fermentation in Staphylococcus, produces lactic acid.
Current Research
One current area of research on the microbial communities present in diabetic foot ulcers is the increasing presence of multi-drug resistant bacteria (MDRB). With the increased use of antibiotics, antibiotic resistance among bacteria has increased, leading to a greater number of infections that are not susceptible to the normal course of antibiotic treatment.
Due to this increasing antibiotic resistance, common types of multi-drug resistant bacteria in diabetic foot ulcers include Methicillin-resistant Staphylococcus aureus, (MRSA) and multi-drug resistant P. aeruginos [11]. Recently, other species in these ulcers have been found to be resistant to different types of antibiotics. These species include E. coli, K. pneumonia, and E. fecalis. MRSA is one of the most prevalent, which is partially due to the fact that it can develop small colony variants (SCVs). The SCVs are able to increase the invasiveness and resistance of the bacteria. They are also smaller than a normal S. aureus cell, which is generally ten times larger than the SCVs. They also have greater resilience against aminoglycosides and those antibiotics that target peptidoglycan within cell walls [2].
Another area of research is to work on characterizing the microbial composition of diabetic foot ulcers using methods other than traditional culture methods. Many past studies, as well as clinics identifying wound communities, have focused on using traditional culture-based methods to characterize the composition of diabetic foot ulcers. These methods generally cannot adequately account for and identify all types of bacteria present, as culture-based methods are estimated to be able to detect 1% of all bacteria [7]. This leads to an overestimation of certain types of bacteria present, such as S. aureus [7].
References
[1] Bowler, P.G., Duerden, B.I., & Armstrong D.G. (April 2001). Wound microbiology and associated approaches to wound management. Clinical Microbiology Review, 14(2), 244-269. doi:10.1128/CMR.14.2.244-269.2001
[2] Cervantes-Garcia, E., Garcia-Gonzalez, R., Reyes-Torres, A., Resendix-Albor, A.A., & Salazar-Schettino, P.M. (2015). Staphylococcus aureus small colony variants in diabetic foot infections. Diabetic Foot & Ankle, 6, 1-5. doi:10.3402/dfa.v6.26431
[3] Dowd, S.E., Wolcott, R.D., Sun, Y., McKeehan, T., Smith, E., & Rhoads, D. (October 3, 2008). Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS ONE, 3(10), 3326. doi:10.1371/journal.pone.0003326
[4] Jurtshuk, P. (1996). Medical Microbiology. The University of Texas Medical Branch at Galveston.
[5] Margolis, D.J., Malay, S., Hoffstad, O.J., Leonard, C.E., MaCurdy, T., Lopez de Nava, K., Tan, Y., Molina, T., & Siegel, K.L. (February 17, 2011). Prevalence of diabetes, diabetic foot ulcer, and lower extremity amputation among Medicare beneficiaries, 2006 to 2008. Data Points Publication Series.
[6] Percival, S. & Bowler, P. (July, 2004). Understanding the effects of bacterial communities and biofilms on wound healing. World Wide Wounds.
[7] Smith, K., Collier, A., Townsend, E.M., O’Donnell, L.E., Bal A.M., Butcher, J., Mackay, W.G., Ramage, G., & Williams, C. (2016). One step closer to understanding the role of bacteria in diabetic foot ulcers: characterizing the microbiome of ulcers. BCM Microbiology, 16(54). doi: 10.1186/s12866-016-0665-z
[8] Stephens, P., Wall, I.B., Wilson, M.J., Hill, K.E., Davies, C.E., Hill, C.M., Harding, K.G., & Thomas, D.W. (March 2003). Anaerobic cocci populating the deep tissues of chronic wounds impair cellular wound healing responses in vitro. British Journal of Demratology, 148(3), 456-466. doi:10.1046/j.1365-2133.2003.05232.x
[9] Todar, K. (2012). Staphylococcus aureus and Staphylococcal disease. Online Textbook of Bacteriology. Retrieved April 25, 2016, from http://textbookofbacteriology.net/staph.html
[10] Uppuluri, P., & Lopez-Ribot, J.L. (February 18, 2016). Go forth and colonize: Dispersal from clinically important microbial biofilms. PLoS Pathogens, 14(2), 1-9. doi:10.1371/journal.ppat.1005397
[11] Xavier, W., Sukumaran, M.T., Varma, A.K., Kumar, H., & Chellan, G. (September 2014). Emergence of multi drug resistant bacteria in diabetic patients with lower limb wounds. Indian Journal of Medical Research, 140(3), 435-437.
[12] Yerat, R.C., & Rangasamy, V.R. (Jul-Sept 2015). A clinicomicrobial study of diabetic foot ulcer infections in South India. International Journal of Medicine & Public Health. 5(3), 236-241. doi:10.4103/2230-8598.161545
[13] 2014 national diabetes statistics report. (2014, October 24). Retrieved March 27, 2016, from Centers for Disease Control and Prevention website: http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html
Edited by Nettie Larson, a student of Mary Beth Leigh at the University of Alaska Fairbanks Template adapted from one used by Angela Kent at the University of Illinois at Urbana-Champaign.