Microbial biofilm inhibits wound healing
Introduction
Following an injury to the skin, such as a laceration or an abrasion, the tissue normally undergoes four basic stages of wound healing. The inflammatory phase initiates the process, during which the body’s immune system sends nutrients, platelets, white blood cells (i.e. polymorphonuclear leukocytes (PMNs) and macrophages), antibodies, enzymes, and other immune factors to the affected area to establish homeostasis and prevent infection. Keratinocytes and inflammatory cytokines are then recruited to the area as the wound enters the epithelialization phase, providing temporary protection and activating the next phase of cell proliferation. Healing wounds form a scab is a result of this epithelialization stage. After a few days, fibroblasts and collagen regenerate tissue and blood vessels in the proliferation stage, until finally reaching the remodeling stage in which collagen is continually formed and lysed until the skin is restored to its original strength.[1]
However, some wounds may be arrested in the inflammatory phase (Figure 1). These wounds, referred to as chronic wounds, may take weeks, months, or years to heal, and become highly susceptible to bacterial infection without persistent care. Chronic wound infections make up 60 - 80% of all human infectious diseases, and are cause of major concern to global health in view of our aging population and increasing prevalence of diabetes mellitus and obesity.[2] One in 20 elderly people live with chronic wounds resulting from diabetes or poor circulation, especially those confined to a wheelchair or a bed.[3] Chronic wounds in diabetic patients can be especially fatal. Every year, chronic wound infections in diabetics lead to over 70,000 lower-leg amputations in the United States alone, and up to half of these patients die within the first 18 months following the procedure. Of those who survive, half lose their contralateral extremity within 5 years.[4] As an estimated 415 million adults currently have diabetes and this number is projected to increase to 642 million by year 2040, developing treatment and prevention methods for chronic wound and infection is and will continue to be crucial to reduce medical costs and improve global public health.[5]
As common as the health problem is, however, current chronic wound treatments are insufficient and too often lead to amputation due to a lack of understanding of the microbiology behind these infections or how to eliminate them. Although it was known that microbial infections significantly complicate and delay wound healing processes, recent research suggests that the presence of microbial biofilms, as opposed to free-swimming planktonic bacteria, contribute significantly to the chronicity of a wound. Over 90% of chronic wounds are infected with biofilms, while biofilms are found in only 6% of acute wounds.[6] The protective and hostile nature of these biofilms renders their complete removal from the wound bed of chronic infections extremely difficult.
These findings lead to a series of intriguing questions. Why do biofilms develop in chronic wounds? Is it the environment of the chronic wound that promotes biofilm formation, or is it the development of a biofilm that causes a wound to become chronic? What are the current methods of treatment of biofilm-associated chronic wounds, and what are the characteristics of biofilms that so often render these treatments inadequate to heal the wound? What are other ways in which researchers and medical providers can explore chronic wound treatment?
This investigation of biofilm formation in chronic wounds will place a special focus on the opportunistic pathogen Pseudomonas aeruginosa and diabetes-associated chronic wounds, as these two areas in microbiology have been well-studied and present good models for relationships between biofilm formation and chronicity of wounds. P. aeruginosa is notorious for its tendency to form resilient biofilms and is the most commonly isolated pathogen from chronic wound infections.[7]
Biofilm General Background
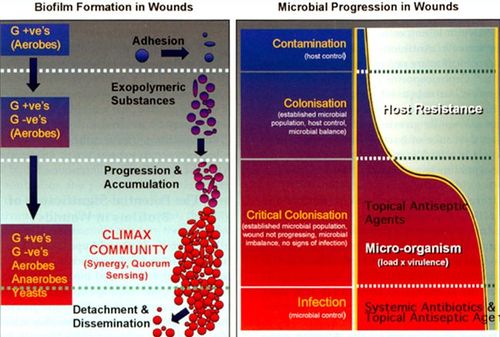
Most bacteria in nature aggregate to form three-dimensional, multicellular communities firmly adhered to a substrate or surface called biofilms. If free-swimming, planktonic bacteria discover a favorable environment abundant in nutrients, they will form sessile biofilms to settle in that favorable environment. A biofilm “life cycle” involves four general stages (Figure 2). The cells adhere to a substrate and form microcolonies, coating the substrate with polysaccharides or glycoproteins for more cells to attach. As more and more cells aggregate, the concentration of chemical signals reaches a point that triggers genetic changes in the cells that cause them to bind tightly to the surface and to neighboring cells. These microcolonies produce a thick extracellular matrix composed of exopolysaccharides (EPS), proteins, extracellular DNA (eDNA), and other polymers that forms a protective physical barrier around the bacteria, allowing them to grow into a mature biofilm of complex communities capable of chemical communication, a process called quorum sensing (QS). QS systems allow bacteria to, in effect, “sense” the presence of other bacteria by their secreted chemical signals. Once the biofilm reaches a particular cell density, a point of saturation, the biofilm turns off the expression of genes producing EPS products and reactivates flagellar motility genes to disperse new planktonic cells from the disseminating biofilm in search of a new environment.[8] P. aeruginosa, for example, produces EPSs such as alginate, Pel, and Psl.[9] When the microcolonies of the biofilm begin to deplete its source of nutrients or oxygen, P. aeruginosa secretes an enzyme alginate lyase that dissolves the EPS in preparation for dispersal.
Microbial biofilms can be found in many environments, such as in the soil, plant roots, the human body, or on industrial materials in wet environments. And not all of these biofilms are pathogenic to the host or surrounding environment; in fact, most biofilm-forming bacteria involved in infection are species of normal nonpathogenic microbial flora, and can even play a protective and beneficial role in the host. Recent research has found that biofilm formation by vaginal lactobacilli provide protection against infectious bacteria.[10] But the balance in microbial composition of nonpathogenic biofilms is fragile; when disturbed, opportunistic pathogens can cause a biofilm to become dangerously noxious. Biofilms are particularly a concern when they develop on medical instruments or devices in the human body during treatments (Figure 3). A biofilm that contaminates an intravascular catheter can cause dispersal cells to be carried into the bloodstream of a patient as it delivers fluids and medications, causing a serious, life-threatening blood infection called septicemia, or sepsis.[11]
It is important to keep in mind that while some biofilms may be predominantly of a single species, most biofilms in nature are polymicrobial, composed of a plethora of different species. The greater special diversity of a biofilm, the better the polymicrobial communities can develop beneficial synergistic relationships to increase its chance of survival.
So why do biofilms frequently develop in chronic wounds and less so in acute wounds? Is it the environment of the chronic wound that stimulates biofilm formation, or is it rather the presence of the biofilm that causes the chronicity of a wound? Studies suggest that the answer is a bit of both - the chronic wound environment is highly hospitable for opportunistic pathogens to form biofilms, and that the properties inherent to biofilms subsequently perpetuates the inflammatory stage in wound healing, prolonging and severely advancing the chronicity of the infection.
Neuropathy is a condition of peripheral nerve damage that develops in 70% of diabetics, causing weakness and/or numbness in the extremities. A diabetic experiencing neuropathy will often suffer an injury to the skin tissue, and, unable to feel or see the injury (especially in the plantar region of the foot), the patient will fail to detect the wound before it becomes chronic.[4] The necrotic tissue and debris that develops in the wound bed before its detection provide the ideal substrate for bacteria to attach and form biofilms. As the wound goes undetected, the accumulation of bacteria further impedes the wound’s ability to heal, inducing the immune system to prolong the inflammatory stage. The interaction of the biofilm with the patient’s immune system perpetuates the chronicity of the wound in a vicious cycle, and the physical and chemical protective properties of the biofilm complicate its removal and prevent antibiotics from penetrating its surface. Moreover, improper or inadequate chronic wound treatment can quickly increase the biofilm’s resistance to them. The following sections will discuss the physical and chemical properties of the interaction between microbial biofilms, antibiotics, and host immune system components in a chronic wound, and describe current detection and treatment methods and assess the advantages and limitations of each.
Biofilms Delay Wound Healing
Three main properties of biofilms contribute to the chronicity of wound infections. First, the diversity of species involved in pathogenic biofilms make it increasingly challenging for finding the appropriate and most effective treatment of chronic wounds, as species that are typically nonpathogenic and susceptible to antibiotics gain antibiotic resistance under the protection of the biofilm and neighboring resistant species. Second, the structure and composition of the biofilm extracellular EPS matrix acts as a barrier against penetration by immune cells and antibiotics, as well as facilitating rapid resistance to debridement (mechanical removal) and antibiotics through chemical-signaling and DNA-sharing mechanisms of quorum sensing and horizontal gene transfer. The matrix also inhibits the healing process by preventing key wound healing components such as fibroblasts and keratinocytes from entering the wound bed. And third, the biofilm’s durable and persistent presence causes the overstimulation of immune responses that, in turn, provide nutrients to the bacteria of the biofilm for further growth and damages nearby, healthy tissue. As long as the biofilm remains, the immune system continually tries to remove the biofilm and perpetuates the inflammatory phase. These unique properties and composition of microbial biofilms thus require their early detection and identification in wounds to develop a customized treatment plan that combines different therapies most effective on the particular biofilm.
Pathogenic Synergy in Polymicrobial Biofilms
Microbial studies of pathogenic bacteria in biofilms and chronic wounds have traditionally placed a heavy focus on the 2% of well-known pathogens that are easily cultured using standard culture methods, and may significantly bias and overestimate conclusions of significance or pathogenicity of these bacteria in chronic wounds if solely drawn from these results.[12] But current research has begun to illuminate the diversity in bacterial species in chronic wounds and how the polymicrobial character of biofilms contributes to their chronicity. Determining the bacterial profile of chronic wounds may aid early detection and treatment of wounds that have the potential to become chronic and prevent development of biofilms before they are able to mature.
The composition of microbial biofilms in species diversity and population appears to undergo a patterned progression over time, and this progression has been paralleled to the model of a progressing biofilm (Figure 4).[13] In the initial phase of wound contamination, before the wound has become chronic, normal skin microbial flora are predominant, mostly consisting of aerobic, Gram-positive bacteria. At this point, the biofilm is not particularly pathogenic, and the activated host immune system will likely heal the wound in a few weeks time. However, facultative anaerobic Gram-negative species may begin to colonize the wound, taking advantage of the niche the oxygen-consuming aerobic bacteria have created for the anaerobes in the wound bed. The balance between microbes and host immune cells are disrupted, and the wound reaches a point of “critical colonization.” As the wound deteriorates further and affects deeper structures such as muscle and bone, more anaerobes are able to burrow themselves deeper into the hypoxic environment of the wound.
The symbiotic relationship between anaerobic and aerobic bacteria in chronic wounds, through a process known as coaggregation, is an example of pathogenic synergy. Coaggregation is a mechanism by which bacteria in biofilms actively attempt to become polymicrobial, recognizing neighboring, genetically distinct species and facilitating cell-to-cell adhesion for metabolic advantages.[14] Obligate and facultative anaerobes take advantage of the favorable hypoxic environment created by the oxygen-reducing aerobes in the biofilm. In chronic wounds, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella spp., Enterobacter spp., and β-hemolytic Streptococcus species are the most cited aerobes, and the most common anaerobes observed are Bacteroides fragilis, Clostridium perfringens, Porphyromonas spp., Peptostreptococcus spp., and Prevotella spp.[15]
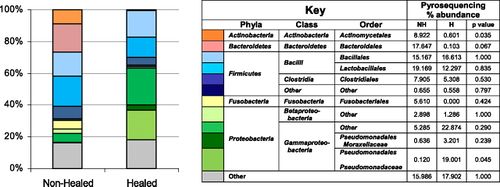
Studies show that biofilms in wounds that heal within a normal time frame have less species diversity, whereas wounds that are slow-healing or non-healing have a higher proportion of anaerobic species and greater diversity of aerobic species (Figure 5).[16][12] This makes sense in terms of survivability – the more diverse the biofilm, the greater the gene pool and the more opportunities for bacteria to establish beneficial synergetic relationships.
The microbial progression in wounds from acute to chronic is a quiet, seamless continuum and varies with the severity of the wound, the degree of immunodeficiency in the patient, and simply by chance exposure of the wound to different environments. It can be challenging to detect whether and at what point a wound will become chronic. More research is needed to establish what species are pathogenic and actively delay wound healing and which are benign to efficiently target and treat biofilms. Some skin microbes may even aid the healing process.[17]
Besides anaerobe-aerobe synergy, polymicrobial biofilm environments provide numerous other advantages, such as the sharing of antibiotic resistance factors and quorum sensing systems, and more efficient DNA-sharing through a greater gene pool. Enhanced resistance in biofilms is not gene-related – the bacteria themselves do not develop resistance, for once dislodged from the biofilm the planktonic bacteria are still susceptible to antibiotics. However, bacteria with previously no resistance to an antibiotic become less susceptible within the polymicrobial biofilm. The following sections describe how this is made possible by the composition of biofilm matrix.
The EPS Matrix: A Barrier to Antibiotics
Bacteria that form biofilms can be 500 to 5,000 times more resistant to antibiotics than planktonic bacteria, including those species in the community who typically are susceptible to that antibiotic in their single-celled form.[7] How does the formation of a biofilm make this possible? There are several ways in which biofilms can be resistant.
First, bacteria in biofilms have reduced growth and metabolic rates because of competition for nutrients and space in the biofilm environment and a reduced need for fast growth due to the association and sharing of metabolic processes among different species.[18] In addition, in expending less energy towards bacterial growth, the bacteria can redirect this energy to produce and strengthen the EPS matrix of the biofilm. Antibiotics, however, are more effective in targeting and killing cells that are metabolically active with high growth rates, as they often target the metabolic pathways and cellular components involved in cell wall biosynthesis.[19] This reduction in growth rate of microbial biofilms renders them less susceptible to antibiotics. Second, the protein and polysaccharide components of the EPS matrix can slow penetration of antibiotics through the biofilm, allowing most mature cells deep within the matrix the time to develop resistance. P. aeruginosa, in example, often mutates into a pathogenic biofilm-producing strain in which the exopolysaccharide alginate is overproduced and results in a mucoid phenotype, increasing its resistance to antibiotics and phagocytosis by host immune cells.[20] The sheer volume of the biofilm matrix alone can be enough to slow penetration by most disinfectants or hinder ingestion by phagocytic cells. And third, some bacteria are intrinsically resistant and produce resistance factors in response to antibiotics that can enhance the resistance of the entire community; this is called passive resistance.
Antibiotic Resistance by Horizontal Gene Transfer
Bacteria can share antibiotic resistance genes by horizontal gene transfer, a mechanism of genetic exchange between different species. In the biofilm, the most common form of horizontal gene transfer is transformation and conjugation. In such a dense environment with a high population count, bacteria in biofilms are more likely to come across and take up extracellular DNA (eDNA) released from its dying neighbors, a process called transformation. Bacteria can use eDNA for nutrients, genome repair, or incorporate into their genome for evolutionary advantage. In a study of chronic wounds in diabetic mice, eDNA was shown to be an integral component of the EPS matrix in P. aeruginosa and increased its tolerance to the antibiotic gentamicin.[7] Alternatively, the dense environment of the biofilm also allows for highly efficient conjugation, where a bacterium with a fertility factor (F+) can donate plasmid DNA to another cell without the fertility factor (F-) through cell-to-cell contact (Figure 6). Greater diversity in the biofilm allows for a greater gene pool for the bacterial community to share through conjugation. Genes encoding for extended-spectrum β-lactamases are a common mobile genetic element that is shared easily by conjugation. β-lactamases are enzymes produced by bacteria that have developed resistance to β-lactam antibiotics such as penicillin, and act against these antibiotics by hydrolyzing the conserved four-atom β-lactam ring structure. When a β-lactam-producing strain of Haemophilius influenza was co-cultured with Streptococcus pneumonieae that lacked any antibiotic resistance factors, S. pneumonia became resistant to amoxicillin.[21]
Quorum Sensing: Resistance to Host Immune Defenses
Perhaps one of the most important and yet least understood mechanisms of resistance in biofilm-forming bacteria is a system of chemical signaling and communication called quorum sensing (QS). QS regulates biofilm development and virulence factor production that increases microbial resistance to the host immune system defenses, its efficiency is enhanced by the structure of the biofilm matrix that allows for high cell density in close proximity.
Research has discovered that QS is a critical determinant of the chronicity of a wound. Acute and chronic wounds express opposite levels of QS regulation: in P. aeruginosa in acute wounds, QS upregulates protein-degrading proteases and downregulates the EPS alignate, allowing the biofilms dispersal or degradation by host immune systems or antibiotics. The wound is allowed to progress normally through the wound healing stages. However, in chronic wounds, the QS system in P. aeruginosa was mutated or downregulated, and the number of these mutants increased as the chronic infection progressed.[9] These mutants may have permanently deactivated QS systems and have lost the ability to disperse, increasing biofilm growth in chronic wounds.
In addition to biofilm development, QS also regulates the production of virulence factors, which are molecules produced by pathogens that contribute to its invasiveness, enabling colonization, immunoevasion, and immunosuppression. P. aeruginosa has a unique, complex QS system that serves as an excellent model of QS-regulated virulence factor production.
P. aeruginosa has three QS systems arranged in a complex, hierarchical manner, in which three key QS signaling molecules, 3-oxo-C12-homoserine lactone, C4-homoserine lactone, and a quinolone called Pseudomonas quinolone signal (PQS) are controlled by systems LasI, Rhl, and Pqs, respectively (Figure 7).[22] These three systems coordinate to produce virulence factors such as elastase, exotoxin A, and pyocyanin. Elastase, controlled mainly by the LasI system, has been found to kill host immune cells such as PMNs by damaging tight-junction-associated proteins and cleave human collagens, which are essential building blocks to tissues.[23] As elastase breaks down the tight-junction-associated proteins, the exotoxin A produced by P. aeruginosa appears to render the cells incapable of restoring them by inhibiting protein synthesis.[24] Pyocyanin acts to increase the production and release of elastase and, if it enters an epithelial cell, causes oxidative damage by synthesis of free radicals.[22]
The components of the EPS matrix regulated by the QS system can also become mutated to produce virulence factors. A key, regulated component of the EPS matrix in P. aeruginosa are rhamnolipids, which are amphipathic glycolipids that usually act as biosurfactants and cause the detachment of the biofilm during the dispersal phase of the biofilm life cycle. Studies have found that QS-regulated rhamnolipids in P. aeruginosa biofilms, specifically rhamnolipid B, damaged PMN plasma membranes, but when the P. aeruginosa QS system was deactivated, PMN necrosis was not observed in the biofilm. This was observed in in vivo and in vitro experiments.[25] Although rhamnolipids usually aid dispersal of biofilms, a mutated QS system can cause it to become a rhamnolipid “shield” against front-line host immune defense cells like PMNs.
Bacterial QS systems of biofilms can do more than conduct the lysing of host immune cells. The host’s own defense system during the inflammatory phase of wound healing can delay the healing process. When the PMNs respond to the presence of bacteria in biofilms, the cells release reactive oxygen species and proteinases called matrix metalloproteases (MMPs) that serve to degrade and dislodge the biofilm matrix. However, as the biofilm persists and the attack launched by the PMNs is prolonged, overstimulation of these oxygen species and proteinases can cause collateral damage to normal, healthy tissue.[25] MMPs degrade proteins by hydrolyzing peptide bonds, and their concentrations must be coupled and balanced with tissue inhibitors of metalloproteinases (TIMPs). In normal or acute wound healing, there is a balance between levels of MMPs and TIMPs at the wound site, but in chronic wounds, this balance is disrupted. Chronic wound infections express increased levels of MMPs and reduced levels of TIMPs, which in turn reduces levels of growth factors needed for further wound repair.[26] A vicious cycle continues as the rhamnolipids (i.e. rhamnolipid B) lyse PMNs found in close contact, leaving the biofilm intact, the PMNs continue to recognize and detect its presence and continue to send more cells to the site. These cells continually release proteinases and lyse, prolonging the inflammatory phase of the wound as well as providing the biofilm with nutrients from the lysed immune cells. As research continues to reveal the role and depth of QS involvement in the pathogenicity of biofilms and chronicity of wounds, scientists are creating QS blockers as a potential and improved treatment method for biofilm-associated chronic wounds.
Conclusion
Current methods of treatment of biofilm-associated chronic wounds are many, but scientists and medical providers have yet to discover the most effective and efficient combination of treatments. There are three main classes of treatments: mechanical debridement, antibiotic treatment, and anti-biofilm/biofilm-disrupting agents such as QS blockers. None of these three classes alone can provide adequate treatment. Mechanical debridement, often by surgical removal, has been frequently found to be insufficient for the complete removal of the biofilm as the EPS matrix burrows into deep tissue structures. The biofilm will also revive from any residue that remains. Furthermore, debridement requires a consistent and extensive (often indefinite) schedule, for wounds debrided at irregular intervals only healed 25% of the time, as compared to 83% of wounds that went on to heal when debrided weekly.[6] Antibiotic treatment alone can also be inadequate since the biofilm has numerous mechanisms of antibiotic resistance, as described in this paper. Combining antibiotic and mechanical debridement methods, on the other hand, does show some promise, but the key is to target early formation – the earlier, the more effective. Research suggests that newly formed biofilms are more susceptible to antibiotics than at later stages in development. After debridement, biofilm-producing bacteria S. auerus and P. aeruginosa were more susceptible to antibiotics in the first 48 hours of biofilm development, but within those 48 hours bacteria were already beginning to develop resistance in the latter half (at 24 and 48 h) as compared to the first half (6 and 12 hours) to 100% bleach and gentamicin – after 72 hours, P. aeruginosa reached its predebridement level of resistance.[27] This combinatorial method, however, still has some limitations. As aforementioned, complete removal by debridement is nearly impossible, and consistent antibiotic treatment of the residue bacteria (likely to be a remnant of the older, mature biofilm) that remain may cause them to develop antibiotic resistance.
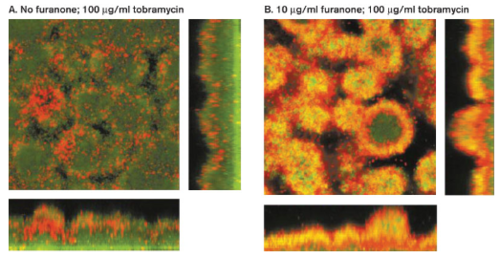
Many researchers are studying antibiofilm agents as an alternative method to antibiotics to treat biofilms. Antibiofilm agents are not antibiotics but affect biofilm development by blocking QS systems, degrading the EPS matrix, or blocking initial attachment of planktonic bacteria. Furanone is one such agent. Furanone is a QS blocker that interferes with the chemical signal molecule acyl homoserine lactone (AHL) responsible for increasing resistance to antibiotics in biofilm communities. When P. aeruginosa was treated with furanone and tobramycin, shutting down the QS system caused the bacteria to become more sensitive to the antibiotic (Figure 8). The QS blocker itself did not weaken or kill the cells directly, but it did increase their susceptibility to tobramycin.[8] For this reason, QS blockers must follow with antibiotic treatment so that the cells do not develop a mechanism of resistance to the blockers.
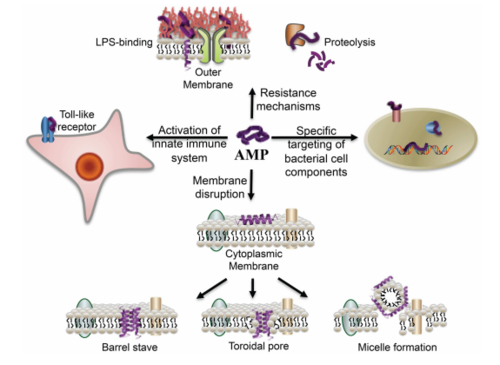
Antimicrobial peptides as anti-biofilm agents have also shown very promising results. In particular, the human cathelicidin antimicrobial peptide LL-37 was shown to have biofilm-prevention, biofilm-disrupting and wound-healing properties when topically applied.[28] Antimicrobial peptides are human immune defense molecules that inhibit cell wall, nucleic acid and protein biosynthesis, but bacteria have not yet developed a resistance to these molecules (Figure 9). These amphipathic molecules are antimicrobial against both Gram-negative and Gram-positive bacteria, inserting itself into their bacterial membrane and forming pores that cause the cell to lyse. By stimulating the expression of type IV pilus biosynthesis genes and increasing bacterial motility, LL-37 prevents the planktonic bacteria from settling and attaching to any substrate surface. It also blocks and downregulates the Las and Rhl QS systems, disrupting biofilm formation in vitro of both P. aeruginosa and S. aureus at concentrations lower than that normally required of antibiotics. Perhaps the most valuable effect of LL-37 is that it protects human keratinocytes from apoptosis and stimulates their migration into the wound site for tissue repair – diabetic mice exhibited vastly improved wound healing with LL-37 gene transfer.
As with any treatment, LL-37 treatment does have some limitations. Controlling the concentrations of LL-37 for the desired effect is challenging, for too high of concentrations caused apoptosis of epithelial smooth T cells, and concentrations too low increased neutrophil survival, which may be problematic with pathogens like P. aeruginosa capable of lysing PMNs like neutrophils, resulting a buildup of inflammatory debris and potential nutrient for the pathogen in the wound bed. However, the study suggests that topical application of LL-37 overcomes these limitations.
In order for these treatments to have their maximum effect, however, the bacterial profile of biofilms must be detected as early as possible. Standard culturing methods take days to obtain results and often times are incapable of detecting microorganisms that demand specific growth conditions like anaerobes, which make up a majority of chronic wound biofilms.[12] Molecular diagnostic methods are needed to detect the biofilm composition within the short time frame available for treatment before the biofilm matures. Studies suggest polymerase chain reaction (PCR) and sequencing to identify bacterial species and quantify their proportions within biofilm communities. Tuttle et. al in their study uses the Ibis T5000 universal biosensor and tag-encoded FLX amplicon pyrosequencing (bTEFAP) to analyze samples of chronic wounds in diabetic ulcers for its combined ability to identify species present at 0.02% or above the total population in the sample, its rapid and high throughput, and low cost per sample.[12] Another suggested and frequently used method is diagnostic tool called “bar coding,” which uses chronic wound fluid samples or tissue biopsies to identify chronicisation markers, such as growth factors and their receptors or MMPs. The growth factor is a practical marker as it is critical in initiating wound healing and diminished levels of this marker is a strong indicator of the chronicity of the wound.[15]
Essential to all of these diagnostic and treatment methods is a greater understanding and distinction of those bacterial species that harm and those that aid wound healing. Tuttle et. al characterization of the microbial composition of chronic wounds revealed that P. aeruginosa, despite its well-known and well-studied pathogenicity, was in fact the predominant species in wounds that healed within 6 months (as compared to Actinomycetales predominant in wounds that had not healed in 6 months time).[12] These results may suggest that P. aeruginosa could act to prevent other, more pathogenic species from colonizing the wound, more or less “protecting” the host from development into a chronic wound infection. Research should place emphasis on the characterization of pathogenic and “beneficial” bacteria in biofilms, and seek the most effective combination of debridement with biofilm-disrupting and/or antibiotic therapies after early molecular diagnosis of the biofilm.
References
- ↑ Assessment Technologies Institute of Nursing Education. “Wound Care: The anatomy and physiology of wound healing.”
- ↑ Dowd, Scot E., Randall D. Wolcott, Yan Sun, Trevor McKeehan, Ethan Smith, and Daniel Rhoads. “Polymicrobial Nature of Chronic Diabetic Foot Ulcer Biofilm Infections Determined Using Bacterial Tag Encoded FLX Amplicon Pyrosequencing (bTEFAP).” 2008. PLoS ONE 3(10): e3326. DOI: 10.1371/journal.pone.0003326.
- ↑ Paddock, Catharine. “Bacteria living on skin may affect how wounds heal.” May 2, 2014. Medical News Today.
- ↑ 4.0 4.1 Watters, Chase, Katrina DeLeon, Urvish Trivedi, John A. Griswold, Mark Lyte, Ken J. Hampel, Matthew J. Wargo, and Kendra P. Rumbaugh. “Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice.” 2013. Medical Microbiology and Immunology 202: 131-141. DOI: 10.1007/s00430-012-0277-7.
- ↑ “IDF Diabetes Atlas Executive Summary.” 7th edition. 2015. International Diabetes Federation.
- ↑ 6.0 6.1 Attinger, Christopher, and Randy Wolcott. “Clinically Addressing Biofilm in Chronic Wounds.” 2011. Advances in Wound Care, 1(3): 127-132. DOI: 10.1089/wound.2011.0333.
- ↑ 7.0 7.1 7.2 Watters, Chase, Jake A. Everett, Cecily Haley, Allie Clinton, and Kendra P. Rumbaugh. “Insulin Treatment Modulates the Host Immune System To Enhance Pseudomonas aeruginosa Wound Biofilms.” 2014. Infection and Immunity 82(1): 92-100. DOI: 10.1128/IAI.00651-13.
- ↑ 8.0 8.1 Slonczewski, Joan L., and John W. Foster. Microbiology: An Evolving Science; Chapter 4 Bacterial Culture, Growth, and Development. 2013.
- ↑ 9.0 9.1 Joo, Hwang-Soo and Michael Otto. “Molecular basis of In Vivo Biofilm Formation by Bacterial Pathogens.” 2012. Chemistry & Biology 19: 1503-1513. DOI: 10.1016/j.chembiol/2012.10.022.
- ↑ Ventolini, Gary. “Vaginal Lactobacillus: biofilm formation in vivo – clinical implications.” 2015. International Journal of Women’s Health 7: 243-247.
- ↑ Donlan, Rodney M. “Biofilm Elimination on Intravascular Catheters: Important Considerations for the Infectious Disease Practitioner.” 2011. Clinical Infectious Diseases 52(8): 1038-1045. DOI: 10.1093/cid/cir077.
- ↑ 12.0 12.1 12.2 12.3 12.4 Tuttle, Marie S., Eliot Mostow, Pranab Mukherjee, Fen Z. Hu, Rachael melton-Kreft, Garth D. Ehrlich, Scot E. Dowd, and Mohmoud A. Ghannoum. “Characterization of Bacterial Communities in Venous Insufficiency Wounds by Use of Conventional Culture and Molecular Diagnostic Methods.” 2011. Journal of Clinical Microbiology 49(11): 3812-3819. DOI: 10.1128/JCM.00847-11.
- ↑ Percival, Steven L., and Philip G. Bowler. “Biofilms and Their Potential Role in Wound Healing.” 2004. Wounds 16(7).
- ↑ Hill, Katja E., Sladjana Malic, Ruth McKee, Tracy Rennison, Keith G. Harding, David W. Williams, and David W. Thomas. “An in vitro model of chronic wound biofilms to test wound dressings and assess antimicrobial susceptibilities.” 2010. Journal of Antimicrobial Chemotherapy: 1-12. DOI: 10.1093/jac/dkq105.
- ↑ 15.0 15.1 Bertesteanu, Serban, Stefanos Triaridis, Milan Stankovic, Veronica Lazar, Mariana Carmen Chifiriuc, Mihaela Vlad, and Raluca Grigore. “Polymicrobial wound infections: Pathophysiology and current therapeutic approaches.” 2014. International Journal of Pharmaceutics 463: 119-126. DOI: 10.1016/j.ijpharm.2013.12.012.
- ↑ Dowd, Scot E., Randall D. Wolcott, Yan Sun, Trevor McKeehan, Ethan Smith, and Daniel Rhoads. “Polymicrobial Nature of Chronic Diabetic Foot Ulcer Biofilm Infections Determined Using Bacterial Tag Encoded FLX Amplicon Pyrosequencing (bTEFAP).” 2008. PLoS ONE 3(10): e3326. DOI: 10.1371/journal.pone.0003326.
- ↑ “Skin Bacteria Could Aid Wound Healing.” Advanced Tissue, May 21, 2014.
- ↑ Jefferson, Kimberly K. “What drives bacteria to produce a biofilm?” 2004. FEMS Microbiology Letters 236(2): 163-173. DOI: 10.1111/j.1574-6968.2004.tb09643.x
- ↑ Ito, Akinobu, Asami Taniuchi, Thithiwat May, Koji Kawata and Satoshi Okabe. “Increased Antibiotic Resistance of Escherichia coli in Mature Biofilms.” 2009. Applied and Environmental Microbiology 75(12): 4093-4100. DOI: 10.1128/AEM.02949-08.
- ↑ Ryall, Ben, Marta Carrara, James E. A. Zlosnik, Volker Behrends, Xiaoyun Lee, Zhen Wong, Kathryn E. Lougheed, and Huw D. Williams. “The Mucoid Switch in Pseudomonas aeruginosa Represses Quorum Sensing Systems and Leads to Complex Changes to Stationary Phase Virulence Factor Regulation.”2014. PLoS One 9(5): e96166. DOI: 10.1371/journal.pone.0096166.
- ↑ Weimer, Kristin E.D., Richard A. Juneau, Kyle A. Murrah, Bing Pang, Chelsie E. Armbruster, Stephen H. Richardson, and W. Edward Swords. “Divergent Mechanisms for Passive Pneumococcal Resistance to b-Lactam Antibiotics in the Presence of Haemophilus influenza.” 2011. The Journal of Infectious Diseases 203(4): 549-555. DOI: 10.1093/infdis/jiq087.
- ↑ 22.0 22.1 Khalifa, Anis Ben Haj, Didier Moissenet, Hoang Vu Thien, and Mohamed Khedher. “Les facteurs de virulence de Pseudomonas aeruginosa : mécanismes et modes de régulations” (Virulence factors in Pseudomonas aeruginosa: mechanisms and modes of regulation). 2011. Annales de Biologie Clinique 69(4): 393-403. DOI: 10.1684/abc.2011.0589.
- ↑ Tamura, Yutaka, Shoko Suzuki, and Takuo Sawada. “Role of elastase as a virulence factor in experimental Pseudomonas aeruginosa infection in mice.” 1991. Microbial Pathogenesis 12(3): 237-244. DOI: 10.1016/0882-4010(92)90058-V.
- ↑ Azghani, A.O. "Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A." 1996. American Journal of Respiratory Cell and Molecular Biology 15(1):132-40. DOI: 10.1165/ajrcmb.15.1.8679217
- ↑ 25.0 25.1 Jensen, Peter Ø., Thomas Bjarnsholt,2Richard Phipps, Thomas B. Rasmussen,2 Henrik Calum,Lars Christoffersen, laus Moser, Paul Williams, Tacjana Pressler, Michael Givskov, and Niels Høiby. “Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensingcontrolled production of rhamnolipid by Pseudomonas aeruginosa." 2007. Microbiology 153(5): 1329-1338. DOI: 10.1099/mic.0.2006/003863-0.
- ↑ Zhao, Ge, Marcia L. Usui, Soyeon I. Lippman, Garth A. James, Philip S. Stewart, Philip Fleckman, and John E. Olerud. “Biofilms and Inflammation in Chronic Wounds.” 2013. Advances in Wound Care 2(7): 389-399. DOI: 10.1089/wound.2012.0381.
- ↑ Wolcott, R.D., K.P. Rumbaugh, G. James, G Schultz, P. Phillips, Q. Yang, C. Watters, P.S. Stewart, and S.E. Dowd. “Biofilm maturity studies indicate sharp debridement opens a time dependent therapeutic window.” 2010. Journal of Wound Care 19 (8): 320-328. DOI: 10.12968/jowc.2010.19.8.77709.
- ↑ Duplantier, Allen J. and Monique L. Van Hoek. “The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds.” 2013. Frontiers in Immunology 4(143): 1-14. DOI: 10.3389/fimmu.2013.00143.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.