Lactose Tolerance
Introduction
Lactose tolerance is the ability to digest the sugar lactose, which is predominantly found in dairy products. This property is made possible via the enzyme lactase, which converts lactose into the easily digestible sugars glucose and galactose via hydrolysis. While nearly all humans are born lactose tolerant at birth. This allows the developing child to feed on breast milk while it develops. Most often, this tolerance erodes over time as the individual matures. This absence of lactase results in lactose malabsorption, which can lead to an array of gastrointestinal symptoms known as lactose intolerance. However, certain populations have evolved lactase-persistence phenotypes and thus, continued lactose tolerance. This allows for these lactose tolerant populations to access a source of nutrition, most often dairy-based in nature, that would otherwise be unavailable to them. Therefore, it is unsurprising that areas that have historically possessed fewer non-dairy caloric alternatives house populations that have the highest amounts of selective pressure for lactose tolerance and therefore higher rates of lactose tolerance. According to best estimates, nearly sixty-seven percent of all humans globally are unable to digest lactose whereas in countries historically more dependent on dairy products such as Denmark, the rate of lactose malabsorption can be as low as four percent.
Section 1 Genetics of Lactase Persistence
Include some current research, with at least one image.
Sample citations: [1]
[2]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
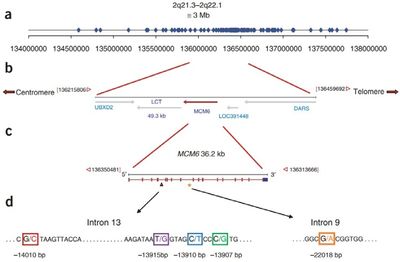
Many times throughout the course of human evolution, the Lactase-permanence mutation (LCT) has arisen. This mutation causes the retention of lactase production beyond the young age where humans would typically stop producing it. This Lactase permanence mutation, while resulting in a single phenotype, can arise from a wide range of separate SNP's (Single Nucleotide Polymorphisms). What all of these most prevalent lactose permanence enabling SNPs hold in common is that they result in constitutive mutations to the LCT genes.That is to say, lactase is always produced in lactase-persistent individuals, without any variance in production due to increased or decreased lactose uptake. If partial lactase-persistence is brought about by a mutation, it may or may not result in a lactose intolerant phenotype. Less than fifty percent lactase activity is often a useful benchmark in determining function. Below that level of activity, an individual will most likely manifest the symptoms of lactose intolerance. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6669050/
The separate incidences of LCT mutants that were positively selected vary by region and ethnicity. These range in age from 8,000-9,000 years ago (certain European populations) to 2,700-6,000 years ago (certain African populations). Somewhat unsurprisingly, these windows of time tend to correlate with the rise of widespread domesticated animal usage in each region. Each of these individual SNP mutations, however, tend to vary in site and function quite widely. For instance, perhaps one of the most prevalent genotypes for lactase persistent individuals in European populations is located upstream from the LCT gene. This upstream genes, known as MCM6, is an important regulator of the LCT gene. The SNP mutation that can occur at that site, known as CT-13910, involves two cytosines (CC) which are replaced with two thymines (TT) during a replication error. This results in a constitutive promoter, and thus, lactase persistence. Another unique example is that of T/G-13915, which is an SNP which results in a similar outcome. Instead of European populations, it is most prominent in populations located on the Arabian Peninsula. (Forsgard)
Interestingly, wherever these unique SNP mutations may emerge, these traits are very heavily retained in populations that consume milk. This suggests that even in areas that have alternative viable caloric sources, the additional calories that dairy represent are significant enough to be selected for nonetheless.
Section 2 Microbiome
While the lactase permanence phenotype is thought to result from a constitutive promoter, meaning that the body is physically unable to adjust its lactase production in accordance with consumption, the human microbiome, on the other hand, is seemingly able to adapt to increases in lactose levels. In an experiment done on the effects of increased lactose presence in the diets of people with lactose malabsorption, the amount of lactase-producing microbes steadily rose in proportion to those microbes unable to digest lactose. Curiously, the converse of this phenomenon was also found to be true, which is that reducing the amount of lactose in the diet reduced the proportion of bacteria which possess the ability to produce lactase. This would suggest that the metabolic tradeoff that lactase production presents outweighs the potential utility it may provide an organism to such an extent that it is actively selected against in the microbiome. (Forsgard)
Another relationship between the microbiome and the digestion of lactose is potentially more beneficial to those with lactose malabsorption. Recent research suggests that the composition of a person's microbiome may affect their ability to digest lactose to an extent. Though the sample study is notably small at eight, it seems that, in part, transplanting certain beta-galactosidase expressing cultures (Such as Lactobacillus acidophilus) may, in fact, reduce the severity of the symptoms associated with lactose intolerance. Because of the incredibly early state of research on the topic, it may be too early for any definitive trends or statements to be espoused with much confidence. [3]
In addition to these previous lactase-dependent relationships, bacteria in the gut microbiome also act as the main driver of the symptoms of lactose intolerance. This occurs when lactose is fermented by bacteria native to the small intestine. This can result in the buildup of bacterial colonies, and gases like hydrogen and methane that arise as a result of fermentation. This results in the manifestation of symptoms such as bloating, indigestion, cramps, and flatulence due to excessive amounts of these gases. In fact, a popular way to test for lactose malabsorption is to study the concentration of hydrogen gas that a person emits after consuming lactose. (Deng)
Conclusion
When one refers to the phenomenon of lactase persistence, one refers to the intersection of demographic trends, genetic mutations, and microbiotic concentrations. Each of these factors can influence the differences between lactase-persistence, lactose malabsorption, and lactose intolerance.
Overall text length should be at least 1,000 words (before counting references), with at least 2 images. Include at least 5 references under Reference section.
References
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
- ↑ Rachel Gingold-Belfer et al., F.A. "Use of a Novel Probiotic Formulation to Alleviate Lactose Intolerance Symptoms— a Pilot Study." 2019. Springer Link 6:2634-2637.
Edited by [Daniel Frank], student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2019, Kenyon College.