Methanogens in Hydroelectric Dams
Introduction

By Claire Sears
Hydroelectric dams are often seen as a green, renewable energy solution to combat climate change; however, methanogenic bacteria growing in dam reservoirs produce methane, a greenhouse gas many times more potent than carbon dioxide. Only 3% of US reservoirs are hydroelectric. Others are for protection from floods and water storage. There is relatively little attention given to this source of greenhouse gas emission both in scientific study and in consideration of dam-building. So far, scientists have not had a standard and accurate way to measure emissions, but a study by Deemer et al. in 2016 developed a measurement strategy.[1] This page will discuss the microbes responsible for the emissions from reservoirs and the factors that influence emission-levels, the measurement of emissions, and the implications for climate change and renewable energy innovation.[2]
Introduction
Hydroelectric dams are often seen as a green, renewable energy solution to combat climate change; however, methanogenic bacteria growing in dam reservoirs produce methane, a greenhouse gas many times more potent than carbon dioxide. Only 3% of US reservoirs are hydroelectric. Others are for water storage and protection from floods. There is relatively little attention given to this source of greenhouse gas emission both in scientific study and in consideration of dam-building. So far, scientists have not had a standard and accurate way to measure emissions, but a study by Deemer et al. in 2016 developed a measurement strategy. This page will discuss the microbes responsible for the emissions from reservoirs and the factors that influence emission levels, the measurement of emissions, and the implications for climate change and renewable energy innovation.
Methane
Methane (CH4) is an abundant gas in the atmosphere produced by both natural sources and by human productionCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. As a greenhouse gas (GHG), methane is 25 to 85 times as potent as CO2, depending on the time frame in questionCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Methane lasts in the atmosphere for approximately 10 years under typical conditionsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title, meaning that in a time frame of approximately 20 years, methane has a global warming potential (GWP) 84 times greater than CO2Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. On a 100 year time scale, methane is 28 times as potent as CO2Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Wetlands are the greatest natural source of methane, with smaller contributions from termite digestive processes, volcanoes, ocean vents, and methane hydrate deposits beneath Antarctic and Arctic ice and along continental marginsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The principal human sources of methane in the atmosphere are the production and combustion of coal and natural gas, livestock farming, biomass burning, and waste managementCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Most naturally produced methane can be offset by uptake into methane sinks such as soil where there are methane-oxidizing soil bacteria and methane oxidation by OH^- in the lower levels of the atmosphereCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. However, methane produced by humans increases atmospheric methane more rapidly than it can be offset by sinksCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Additionally, as methane concentration increases, there are fewer methane oxidation radicals (OH^-) available in the environment to oxidize methaneCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. This prolongs the life of methane in the atmosphere and increases its greenhouse effectCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Methane production can also be used as a renewable source of energy by burning methane produced naturally and by human causesCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. This process releases CO2.
TABLE 1 METHANE CYCLE DIAGRAM
Methanogenic Microbes
Methanogenesis Overview
At least 80% of atmospheric methane has been produced by obligately anaerobic methanogenic archaea, the rest being released by natural gas wells and coal depositsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Generally, CO2 is used as the terminal electron acceptor in methanogenesis, while H2 functions as the electron donorCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Methane can also be produced from a few other substances, such as methanol, acetic acid, formic acid, and methylamines. These substances must first be converted to acetate or CO2 and H2 by other anaerobic microbes for use by methanogensCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. By consuming hydrogen gas, methanogens support growth of other anaerobic bacteria in the environmentCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Methanogen Diversity and Taxonomy
Within the Euryarcheota branch of Archaea are several branches of methanogens, microbial producers of methaneCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Methanogens are highly morphologically diverse, possibly displaying as much diversity than the entire bacterial domainCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. There are five major polyphyletic clades of methanogens. Methanosarcinales break down alcohol, acetate, and amines to methane in oceans and soil. Methanobacteriales are found in soil and animal digestive tracts. The other three clades include marine organisms and hyperthermophiles.
Methanogen Habitats and Ecology
Because methanogens can produce energy from only a small range of organic substrates, they generally live in syntrophy with other microbes that produce their required substratesCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
One of the major habitats for methanogens is anaerobic soil found in wetlands. Wetlands producing the most methane are generally those affected by human intervention, such as rice paddies and dam reservoirsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Artificial or disturbed wetlands are likely to have high concentrations of fertilizers and bacteria that convert carbon sources to methanogenic substrates, making these ideal for growth of methanogens. Other common habitats include landfills, Arctic lakes, ocean sediment, and the digestive tracts of animals, especially ruminants like cowsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Rising global temperatures increase the rate of methanogenesisCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Dams and hydroelectric energy sources
Historical use of dams
Man-made dams have been constructed across rivers and streams for centuries to hold back water, creating reservoirsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. A reservoir is an artificial lake. Dams have been used to prevent flooding, to divert water for agriculture and human use, and to generate mechanical power for use in millsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Since the industrial revolution, dams have been used to generate electricity. According to Deemer et al., there are “847 large hydropower projects (more than 100 MW) and 2853 smaller projects (more than 1 MW) that are currently planned or under construction,” so the impact of hydroelectric dams will only continue to growCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Hydroelectric dams
Hydroelectric power is generated by harnessing the potential energy of water with mechanical turbinesCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Usually, hydroelectric power plants use dams across rivers to raise water levels and capture energy as water falls through or over damsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Hydroelectric power is often touted as a green energy source because it is renewable through the hydrologic cycle, and its production does not directly emit greenhouse gasesCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Energy demands fluctuate throughout the day, so water can be pumped to a higher level using excess electricity generated by turbines and allowed to lower at times of peak energy demandCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Ecological consequences
Though hydroelectric power is a renewable energy alternative, it is not without environmental and social consequences. Dams alter habitats and nutrient flows by blocking water and creating reservoirs. A well-known ecological consequence of dams is disruption of fish habitats and migration patternsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. They can also disrupt ecosystems by flooding surrounding areas. In Brazil, hydroelectric dams have become a very popular source of energy and numerous dams have been built on the tributaries of the Amazon River and other major rivers in Brazil, disrupting the lives of indigenous people and tropical rainforest along the riverCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The deforestation of tropical rainforest is destructive to the global environment because it destroys a major carbon sink.
The formation of reservoirs often creates conditions ideal for methanogenesis. The construction of a hydroelectric reservoir can flood areas with large stores of terrestrial organic matter both above and below ground, which fuels microbial decomposition of organic matter to CO2 and other substrates suitable for methanogenesisCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Decreasing water level on shores also leads to an increased CH4 bubbling rate. This means that oxidation processes converting CH4 to the less potent greenhouse gas CO2 in sediments and the water column are bypassed and CH4 is released directly to the atmosphereCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Dam reservoirs tend to have higher “catchment area-to-reservoir surface area ratios and are located on larger streams,” leading to greater loading from water, sediment, organic matter, and nutrients than in natural lakesCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Effect of methanogenesis in dam reservoirs
Emissions
Deemer et al. estimate that on a 100 year timescale, GHG fluxes from reservoirs are responsible for 1.3% of CO2-equivalent emissionsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. CH4 emissions are responsible for 80% of the radiative forcing (what leads to global warming) from reservoirs on a timescale of 100 years and 90% on a timescale of 20 years, meaning that the vast majority of the contribution to global warming made by reservoirs results from methanogenesisCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. In a review of hydroelectric and nonhydroelectric dams, “16% of reservoirs were net CO2 sinks and 15% of reservoirs were net N2O sinks, whereas all systems were either CH4 neutral or CH4 sources”Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The same review also found that, in contrast to popularly held belief, subtropical and midlatitude reservoirs could emit as much CH4 as tropical reservoirs.
The two emission pathways typical of both natural wetlands and dam reservoirs are ebullition (bubbling) and diffusion pathwaysCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. GHG emission pathways that are negligible in natural systems but common in reservoirs include “drawdown emissions, downstream emissions, emissions from decomposing wood, and emissions from dam spillways and turbines”Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Drawdown emissions happen with changing water levels that alter hydrostatic pressure, and therefore alter the release of gases. Changing water levels also vary exposure of sediments to air, which changes redox conditions in sediment, which in turn affects decompositionCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Downstream emissions are emissions of GHGs produced in the reservoir, which pass through the dam and are emitted downstream. Dam spillways and turbines result in “degassing” by releasing gases previously trapped underwaterCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. According to Deemer et al., “both downstream and degassing emissions are likely highly dependent on reservoir GHG concentrations, dam engineering, spill practices and down-stream biogeochemistry”Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. For example, turbines that generate energy from deeper water are likely to emit more GHGs through degassing and downstream emissions because deeper, anoxic water tends to have a higher concentration of GHGs as compared with surface water. GHGs will be trapped in the deep water as the water flows through the turbines and are carried downstreamCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. These sources of emissions are still poorly understood and quantifiedCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Influencing factors
Latitude and reservoir age have been said to predict CH4 and CO2 emissionsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Higher temperatures increase microbial productivity and the amount of available organic matter, so dams at lower latitudes are said to produce more greenhouse gases. Younger reservoirs are thought to have higher GHG production because of the sudden availability of organic matter that decreases over time, though the evidence backing this is contradictoryCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Other studies have highlighted the effect of nutrient availability in reservoirs on GHG emissions. Some posit that increased nutrient availability leads to increased primary production, which makes reservoirs into carbon sinks. Others posit that it leads to eutrophication in which anaerobic conditions develop in reservoirs and dead organic matter increases, leading to an increase in GHG fluxCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Of the characteristics studied by Deemer et al. in a review of emission studies, a higher concentration of chlorophyll a in the water and higher air temperatures were most predictive of greater CH4 emissionCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Chlorophyll a concentration indicates eutrophic statusCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
A study quantifying differences in methane emissions from a subtropical hydroelectric reservoir over a 9.5-year period found seasonal variation in CH4 emissionsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The season with the lowest emission levels was the warm dry season because there was the highest stratification of the water column, which kept gases from lower layers from diffusing across the surface or rising as bubbles. Gases were released more in the warm wet season and even more so in the cool dry season when the water overturns. They also found that locations in the surface of the reservoir with more artificial mixing had higher CH4 emissionsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
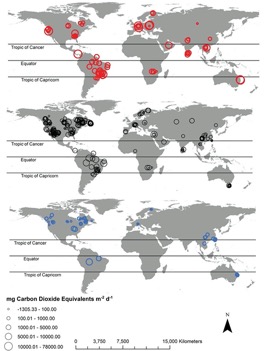
Quantification of emissions from dam reservoirs
Greenhouse gas fluxes from aquatic sources are measured using various techniques, such as “floating chambers, thin boundary methods, eddy covariance towers, acoustic methods, and funnels”Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The problem is that they provide varying levels of accuracy and spatial and temporal coverageCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. In addition, common measurement techniques of aquatic GHG emissions tend to focus on diffusion of GHGs across the water-air interface, which measures CO2 and N2O fluxes well as these gases are readily dissolved in water. However, these measurements combining water-air gas exchange with concentrations of dissolved GHGs fail to capture the extent of CH4 emissions because CH4 is far less soluble in water and tends to be released as bubblesCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Other methods, such as floating chambers, frequently exclude ebullition events because they “interfere with the linear accumulation of CH4 within a sampling chamber”Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Another difficulty is that common techniques for measuring CH4 ebullition, such as inverted funnel traps, typically only collect data over a short period of time and over a relatively low area. Therefore, measurements fail to capture the variability of fluxes over time and spaceCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. New technology has improved the ability of scientists to measure ebullition rates from reservoirs; however, it is not widely used and is often costlyCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. In studies employing these new technologies (eddy covariance and acoustic methods), “mean ebullition + diffusion fluxes were over double that of diffusion-only fluxes,” highlighting how important it is to include ebullition rates in the measurement of aquatic CH4 emissionsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The percentage of CH4 flux from ebullition also varies enormously, making up “0% to 99.6% of total CH4 flux,” so it is important to take both ebullition and diffusion measurementsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Global estimates of GHG flux depend on an accurate estimation of reservoir surface areaCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. According to Deemer et al., “Global Reservoir and Dam Database (GRAND) together with pareto-based extrapolations… estimate that reservoirs more than 0.00001 km2 cover 507,102 km2 of earth’s surface.”Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. There are also regions with limited data on GHG emissions from reservoirs, including Russia, Australia, and AfricaCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Agricultural dams
A study of GHG emissions from small agricultural dams in Australia draws attention to the importance of including emissions from small-scale agricultural dams in global calculation of GHG emissions in addition to large hydroelectric reservoirsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The study measured CH4 flux and CO2 diffusion from 77 small-scale agricultural dams found in southeast Australia. The study found that CO2-equivalent emissions from small agricultural dams were 3.1 times higher than emissions from temperate reservoirs in the state of Victoria, Australia, even though the farm dams covered only 0.94 times the surface areaCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. They also found that nitrite concentration was positively correlated with both CO2 and CH4 emissions and that CH4 emissions were higher for livestock dams than crop-producing damsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Though this study sampled from only a small geographical region, it shows that smaller agricultural dams can be huge producers of GHGs, and this is likely related to the nutrient inputs from livestock and fertilizers. 12 FIGURE 5
Global examples
In 2009, a yearlong study of a 90-year-old reservoir in Switzerland found the highest level of CH4 ever documented for a midlatitude hydroelectric reservoirCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The study measured ebullition and diffusion. Diffusion of methane from sediment was low and did not change seasonally, though dissolution of bubbles was positively correlated with water temperature.
The United States is home to 2198 dams actively producing hydropowerCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. A study of dams in the United States found that GHG emissions were site specific and that GHG emissions from hydroelectric reservoirs were much lower than those in tropical regionsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Diversion dams had lower emission rates than dams with reservoirsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. This makes sense, following the idea that organic matter and nutrient buildup allows for the growth of methanogens beneath the water’s surface. However, as this study was a review of other studies, the findings may be susceptible to bias from the lack of measurement of CH4 ebullition. A study of two hydroelectric reservoirs in eastern Washington found that over 97% of methane emissions came from methane ebullition and that ebullition rates in the summer were higher than those of six hydroelectric reservoirs in the southeastern U.S.Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title.
Hydroelectric power has seen huge popularity and growth in the Amazon basin. In recent years, methane emission from hydroelectric reservoirs has been increasingly studied. Quadra et al. pointed this out and the fact that there has been little investigation into the organic material buried by hydroelectric reservoirsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The study found high levels of organic matter in reservoir sediment. They also found that 23% of sediment samples had dissolved CH4 levels above saturation concentration, “indicating a high potential for CH4 ebullition”Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. The study shows that while organic matter burial in reservoirs could be considered a form of carbon sequestration, it can also mean increased carbon availability for methanogenic microbes operating in anoxic conditions in the sediments of hydroelectric reservoirsCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. 15 FIGURE 5
In China, although hydroelectric dams contribute GHG emissions 2-fold greater than in temperate zones, hydroelectric power produces 7% of the GHGs of a typical coal-fired plant, making hydroelectricity a relatively green alternative to the main source of electricity, according to Li et al.Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. One of the strengths of their study was that it quantified emissions from degassing and drawdown; however, it sourced reservoir surface emissions from other available research, which may have been subject to the lack of proper quantification of CH4 ebullition discussed earlier.
Remediation and solutions
One option for reducing the greenhouse effect of GHGs produced in hydroelectric reservoirs is to capture the methane and use it as an energy sourceCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Since the methane is already being produced, capturing it and using it to produce electricity converts it to CO2, a less potent greenhouse gas, and extends the energetic capacity of a hydroelectric plant. This would mitigate the greenhouse effect produced by existing hydroelectric reservoirs and reduce dependency on fossil fuels.
As nearly every article cited here says, the most important measure is the inclusion of reservoir emissions in global emissions calculations. That would ensure that methane produced and released to the atmosphere by methanogens in hydroelectric dams would be taken into account when assessing possible renewable energy projects. Understanding the conditions that lead to increased methanogenesis in reservoirs may influence design and location choices in order to reduce emissions. Additional knowledge may also lead to decisions to develop other sources of renewable energy that do not produce as many GHG emissions, such as solar or wind energy.
Conclusions
Though there has been a lot of study in recent years on methanogenesis in hydroelectric reservoirs, it still is not widely known that hydroelectric plants are major sources of greenhouse gases, and there is still much to be understood. Studies with a consistent and accurate way to measure methane emissions are still needed. It would also be valuable to develop methods to accurately and cheaply quantify methane emission from degassing and downstream emissions. Quantification of CH4 emissions from regions lacking data would also be tremendously useful both in determining factors that contribute to methanogenic conditions and in calculating overall emissions on a global scale. Deemer et al. emphasize the importance of timescaleCite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title. Methane is an extremely potent greenhouse gas that has the greatest effect in the 10-20 years after emission. Making decisions that reduce methane emissions will have the greatest immediate effect.
References
- ↑ Deemer, B. R.; Harrison, J. A.; Li, S.; Beaulieu, J. J.; DelSontro, T.; Barros, N.; Bezerra-Neto, J. F.; Powers, S. M.; dos Santos, M.A.; Vonk, J. A.; Greenhouse Gas Emissions from Reservoir Water Surfaces: A New Global Synthesis 2016. BioScience, 66:11:949-964.
- ↑ Weiser, M. "The hydropower paradox: is this energy as clean as it seems?" 2016. The Guardian 6:2634-2637.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.
