Bordetella pertussis and the Importance of Vaccination
Introduction and History
By Alexandra White
Vaccination is a widely used practice to help prevent infectious disease and commonly spread illnesses. A widely known and common vaccine is used to prevent infection of Bordetella pertussis, the causative agent of Whooping cough. Whooping cough is known as one of the most common infectious disease deaths in the world[1]. The disease results in over 50 million cases worldwide per year, with the majority being unvaccinated individuals present in Third World Countries[1].
B. pertussis is spread through coughing and sneezing and symptoms first appear seven to ten days after infection[2]. These symptoms include: fever, runny nose, coughing which develops into a whooping cough, and phenomena[2]. Those infected with pertussis are contagious for around three weeks once coughing is displayed as a symptom yet symptoms can last up to eight weeks[2].
B. pertussis was first discovered and isolated to be the cause of whooping cough in 1906 [1].Compared to human infectious diseases pertussis is a relatively recent human infectious disease that first appeared in France in 1414[3].The disease was identified in Paris, France in 1900 by Jules Bordet and Octave Gengou who obtained the sample from a 5-month-old child[3]. The bacteria was finally isolated in 1906 from Jules Bordet’s own son[1]. A vaccine for B. pertussis was developed in the early 1940s [4]. Before the development of the vaccine there were over 200,000 cases in the United States reported annually and with widespread use incidence has decreased 75% compared to the pre-vaccination era[4].
Bordetella pertussis and Infection Stages
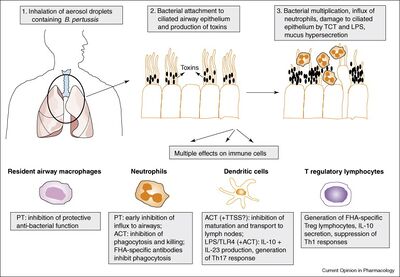
B. pertussis is a gram-negative, aerobic coccobacillus bacteria a part of the genus Bordetella[3]. Pertussis is a human-specific pathogen causing severe respiratory disease that cannot survive outside of its host[3]. Infection occurs with B. pertussis entering through the host airway via respiratory droplets from an already infected individual, usually from coughing [5]. B. pertussis then proceeds down the respiratory tract and adheres to ciliated epithelial cells in the trachea and nasopharynx [5]. Once the bacteria is attached it begins to replicate and colonies with-in the host cells [5]. Toxins are secreted by micro-organism which inflict damage to the epithelial lining, resulting in loss of ciliated cells which induces the coughing most commonly associated with whooping cough [5]. The pertussis toxins released also allow the bacteria to avoid host immune response by interfering with clearance mechanisms [5]. The damage caused by the toxins released by the replicating bacteria also halts ciliary function, short-circuiting host G proteins signaling apparatus, and also inhibits immune cell functions by up regulating cAMP levels [5].
The infection of B. pertussis has an incubation period of seven through ten days but a range of four through 21 days [4]. Infection consist of three stages: catarrhal, paroxysmal, and convalescent[4]. Catarrhal stage starts the onset of symptoms including runny nose, sneezing, and a minor cough [4]. One to two weeks later the cough becomes more severe and the disease progresses into the paroxysmal stage [4]. A fever is either not present or has minimal presence during infection at any stage [4]. The paroxysmal stage is commonly when whooping cough is diagnosed due to numerous, rapid coughs due to thick mucus building up [4]. The end of the paroxysmal stage begins with a high-pitched whoop and difficulty breathing where the infected individual may become cyanotic (blue or purple discoloration due to lack of oxygen)[4]. These symptoms usually occur as attacks rather than continuous symptoms [4]. They occur more often during the night and may reach 15 attacks in 24 hours[4]. This stage may last anywhere from one week to 10 weeks [4]. The convalescent stage consists of a gradual recovery yet paroxysmal symptoms may reoccur and cause respiratory infections for months after diagnosis [4].
B. Pertussis Vaccine History

Soon after B. pertussis was isolated as the causative agent of whooping cough, Pertussis whole-cell (Pw) vaccines were implemented using heat-killed bacteria [3]. Starting in 1914 the first vaccine was licensed in the United States using whole killed bacteria but was used more for treatment than prevention [6]. The problem with early Pw vaccines is they killed whole cell organisms which contained endotoxins that could cause serious side effects[7]. Soon after the Pw vaccine was first discovered, new research and diagnostic tools were used to continue researching for more effective and safe vaccines. Through 1934-1935 Pearl Kendrick and Grace Eldering ran clinical trials on children using a whole-cell vaccine that was generated from chemically inactive bacteria instead of just heat killed bacteria [8]. The vaccine showed an efficacy rate of 89% and was widely distributed [8]. Pw vaccines became used for wholesale distribution starting in the 1940’s [9]. Since B. pertussis in human host restrictive, there is a very low display of genetic diversity [3]. Recent analyses have concluded that there were several different types of isolates circulating in the pre-vaccine era that produce various proteins [3]. Only one or two types predominated with no significant difference in an associated pattern to which was the dominant isolate [3]. This allowed for the Pw vaccine to be composed of 1-3 strains selected from the predominant isolate types [3]. Pw vaccines were given to infants and toddlers extensively due to the majority of infections occurring in younger children [3]. Where vaccine distribution was effective, vaccine strains of B. pertussis were low and in areas with low vaccination rates the pre-vaccine era isolated were still circulating [3]. Pw vaccines were shown to create immunity for some isolates of B. pertussis but not all isolates [3].
By 1943 the American Academy of Pediatrics (ACIP) allowed for routine use in all children [8]. In 1948 the DTP vaccine, which contained diphtheria, tetanus, and whole-cell pertussis vaccine) was created as used effectively[8]. By the 1980s B. pertussis infections had fallen 99% and the fear of infection was replaced into the fear of the vaccine’s side effects[8]. Common side effects included: fever, crying, febrile seizures, limb redness and swelling, and hypo-responsive episodes[8]. The side effects did not cause extreme harm but the public began linking the vaccine to sudden infant death syndrome (SIDS), asthma, and encephalopathies[8]. Through the 1970-8os there was a decrease in vaccination rates due to countries recalling the vaccine due to safety reasons or lack of parental approval [6].
With the decrease of vaccination and the increase of cases, research began developing an effective acellular vaccine containing B. pertussis antigens rather than heat killed bacteria [8]. This vaccine contained formaldehyde-treated filamentous hemagglutinin and formalin-inactivated pertussis toxin which resulted in a 69% efficacy rate[6]. By the 1990s the use of a combined diphtheria, tetanus, and acellular pertussis vaccine (DTaP) became the most common form of the vaccine in the US[8]. To date, the vaccine has continued to be successful but the length of protection has been ambiguous[8]. Most commonly, research has shown additional doses of the vaccine may be necessary to have immunity against the disease [10]. The vaccine showed an efficacy rate of 89% and was widely distributed [8].. The ACIP and the CDC currently recommends a four to five doses of the DTaP with three during infancy and a booster in pre-school age children or in adolescence.
Vaccine Virulence
While B. pertussis vaccines have decreased overall mortality rates by over 90% there is still a current resurgence in whooping cough cases [11]. The two most common reasons for the resurgence of whooping cough is that the aP (acellular whole cell) vaccine has a relative short-term protection rate and the evolution of the b. Pertussis pathogen [11]. Current evolution has included the expansion of B. pertussis strains that contain an allele for the pertussis toxin (ptx) promotor ptxP3 that replaces the ptxP1 strains [12]. PtxP3 strains produce more ptx than the ptxP1 stains which increase the virulence factors of b. Pertussis that increases the pathogens overall fitness[12]. PtxP3 causing b. Pertussis produces more pertussis toxins and suppress the host immune system more effectively than ptxP1[12]. Sarfarchi et al (2016) found that the ptxP3 strains colonized the respiratory tract in vaccinated and unvaccinated mice better than ptxP1 Cite error: Closing </ref> missing for <ref> tag. Recently, there has been an increase in mutations of B. pertussis that are not producing pertactin which is a pertussis acellular vaccine immunogen that is a part of the bacteria adhering to the upper respiratory epithelium [13]. The strains of B. pertussis that do not express pertactin have been increasing in regions where acellular pertussis vaccines have been used for over seven years [13].
Why Vaccination?
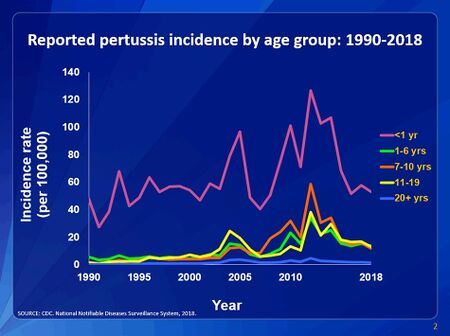
Conclusion
References
- ↑ 1.0 1.1 1.2 1.3 Kerr, J.R. and Matthews, R.C."Bordetella pertussis Infection: Pathogenesis, Diagnosis, Management, and the Role of Protective Immunity." 2000. European Journal of Clinical Microbiology and Infectious Disease 19:77-88.
- ↑ 2.0 2.1 2.2 World Health Organization: Pertussis 2018
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Guiso, N. "Bordetella pertussis and Pertussis Vaccines." 2009. Clinical infectious Diseases 49:1565-1569.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 Havers et al. "Pertussis." 2021. Center for Disease Control and Prevention. 1-16.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Smith, A.M et al. "The virulence factors of Bordetella pertussis: a matter of control" 2001. FEMS Microbiology Reviews 25:309-333.
- ↑ 6.0 6.1 6.2 Klein, N.P et al. "Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers" 2013. Pediatrics
- ↑ Kerr, Cherry J.D."The Epidemiology of Pertussis: A Comparison of the Epidemiology of the Disease pertussis With the Epidemiology of Bordetella pertussis Infection" 2005. American Academy of Pediatrics" 1422-1427.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 Kuchar, E. et al. "Pertussis: History of the Disease and Current Prevention Failure" 2016. Advances in Exerimental Medicine and Biology
- ↑ Mattoo, S. Cherry, J.D. "Molecular Pathogenesis, Epidemiology, and Clinical Manifestations of Respiratory Infections Due to Bordetella pertussis and Other Bordetella Subspecies" 2005. Clinical Microbiology Reviews
- ↑ Gustafsson, L. et al. "Long-term Follow-up Swedish Children Vaccinated With Acellular Pertussis Vaccines at 3, 5, and 12 Months of Age Indicates the Need for a Booster Dose at 5 to 7 Years of Age" 2006. American Academy of Pediatrics. 978-984
- ↑ 11.0 11.1 Dorji, D. et al. "Bordetella pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance." 2018. Medical Microbiology and Immunology 207:3-26.
- ↑ 12.0 12.1 12.2 King A.J. et al. "Genome-Wide Gene Expression Analysis of Bordetella pertussis Isolates Associated with a Resurgence in Pertussis: Elucidation of Factors Involved in the Increased Fitness of Epidemic Strains." 2013. PLOS Computational Biology.
- ↑ 13.0 13.1 Bodilis, H and Guiso, N."Virulence of Pertactin-Negative Bordetella pertussis Isolates from Infants, France" 2013. Emerging Infectious Disease 19:471-474.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
