Naegleria fowleri aka "Brain Eating Ameoba"
Introduction
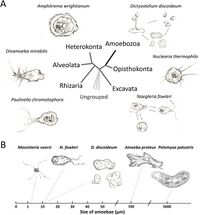
Ameoba are single celled organisms that are found in every major eukaryotic lineage. They are eukaryotic organisms that are defined by their lack of cell walls and the presence of pseudopods. While amoeba exist in many different lineages, the only 'true' amoeba are ones that are in the taxa Amoebozoa.
[2]
They live in the water, soil, and air of a number of diverse ecosystems. They are often predators that consume fungi and bacteria and aid with the recycling of nutrients. They feed via phagocytosis, engulfing smaller particles and organisms into their cytoplasm using their pseudopods. To regulate their internal environment, amoebas have vacuoles that regulate osmosis.
Amoeba can have vastly different structures, which is determined by the actin filaments in the cytoplasm. It is these filaments that control the structure of the pseudopods.
[1]
Amoeba are divided into two main groups: free-living and non-free-living, some of which are pathogenic to humans. One of the most pathogenic species of amoeba is Naegleria fowleri, which belongs to the Naegleria genus. Fortunately only one,the Naegleria fowleri, out of 30 species in this genus can infect humans.
Pathogenic Amoebas
Naegleria fowleri is a highly dangerous ameoba that can infect humans and cause death within 7 to 10 days of infection. This amoeba attacks brain tissue and causes brain swelling; an infection known as primary amebic meningoencephalitis (PAM).
N. fowleri survives best in warm water like hot springs, ponds, and poorly chlorinated pools. They cannot survive in salt water. These are heat loving amoeba and can survive in temperatures up to 46 degree celsius, making them ideal to adapt to internal body temperatures of humans and other animals.
Naegleria fowleri has three different lifeforms — trophozoite, flagellate, and cyst. They are often in their cyst form when environmental conditions are not ideal. This form has an endocyst wall to protect against large changes in pH or temperatures. The flagellate form is pear shaped with two flagella. The trophozoite form is infectious to humans and is when the amoeba eats (via trogocytosis and phagocytosis) and replicates (via binary fission).This form has the characteristic pseudopods, which are used to classify amoeba.
[3]
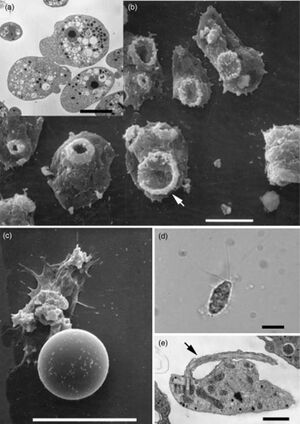
There are a few other pathogenic amoeba that cause diseases in the central nervous system (CNS) such as Balamuthia mandrillaris, Sappinia diploidea, and Acanthamoeba. Infections from Balamuthia mandrillaris and Acanthameoba are fatal, while infection from Sappinia diploidea is non-fatal.[4]
Method of Invasion
Naegleria fowleri enters the human body via the nasal cavity, when contaminated water is inhaled. However, no damage is done to the nasal cavity. Instead most of the damage is found in the olfactory bulb and other regions of the brain, which the trophozoite gains access to by attaching to the nasal mucosa and then traveling through the cribriform plate. It is clear that there is some signal in the brain that the N. fowleri are targeting, but because there are no suitable animal models, discovering what is providing the N. fowleri with this ‘cerebral roadmap’ has been difficult. However, progress has been made identifying acetylcholine as a possible signaling protein. Research done by Abdul Mannan Baig (2016) has discovered homology between acetylcholine and a G-protein coupled receptor on N. fowleri. It could be acetylcholine and other growth promoting chemicals that are signaling the amoeba to target the brain. More research will need to be done to confirm these results.[5]
Symptoms for PAM are very similar to that of bacterial meningitis, so often a PAM diagnosis is not given until after death. The initial symptoms are fever, vomiting, altered sense of smell, and severe headaches, that progress into more violent symptoms such as seizures, stiff neck, coma, hallucinations, and apprehension. People with PAM die an average of 5 days after symptoms begin.
Most of the people who contract PAM are engaging in water related sports, though a few cases have been reported from people who use improperly sanitized nasal rinses.
Immune Response
Once the Naegleria fowleri has entered the brain, the trophozoites cut off and ingest various vital cells such as nerve cells. This damages the brain tissue and causes hemorrhaging, which triggers an inflammatory response, causing further damage to the brain. The innate immunity response involves activation of the complement, neutrophils, and macrophages. Unfortunately, it appears that N. fowleri has begun to acquire immunity to the body’s natural responses, such as avoidance of complement lysis.
The key to surviving infection by Naegleria fowleri is early identification and quick treatment. While there is no official treatment for Naegleria fowleri, a combination of Amphotericin B with rifampin and other antifungal compounds (Seidel et al., 1982). Further research is being done on animals, such as mice, to study the progression and possible treatment of Naegleria fowleri infections.
One study, Yun et al., (2002), that was observing possible genetic differences between pathogenic and non-pathogenic Naegleria found that there were two proteins: high mobility group protein and the 26s proteasome subunit were responsible for increasing pathogenicity of N. fowleri.[6]
Survivors of Naegleria fowleri
From 2012 to 2021, there have been a total of 31 cases of infection by Naegleria fowleri with only 3 survivors.
One such case occured in 2017 to a 12 year old girl in Arkansas who was admitted to the hospital with a high fever, trouble waking up, vomiting, and trouble opening her eyes. A spinal tap was done to rule out meningitis and then Naegleria fowleri was identified to be the cause of the symptoms. She was quickly treated with a round of antibiotics and antifungals: Amphotericin B, Rifampin, Fluconazole, Dexamethasone, and Azithromycin. She was also placed on a catheter to reduce swelling in her brain. Even on this treatment, she continued to deteriorate. In response, she was given Miltefosine and her body temperature was lowered to prevent further brain damage by swelling. She remained in the ICU for 18 days under therapeutic hypothermia before CFS samples could be taken and it was verified that there was no remaining Naegleria fowleri left in her system. While the girl did sustain some brain damage, she was able to make a full neurologic recovery and was able to walk unassisted three months after the infection.[7]
References
- ↑ 1.0 1.1 Shi Y, Queller DC, Tian Y, Zhang S, Yan Q, He Z, He Z, Wu C, Wang C, Shu L.2021.The Ecology and Evolution of Amoeba-Bacterium Interactions. Appl Environ Microbiol87:e01866-20.https://doi.org/10.1128/AEM.01866-20
- ↑ Jeon K, editor. The biology of amoeba. Elsevier; 2012 Dec 2.
- ↑ [Francine Marciano-Cabral, Guy A. Cabral, The immune response to Naegleria fowleri amebae and pathogenesis of infection, FEMS Immunology & Medical Microbiology, Volume 51, Issue 2, November 2007, Pages 243–259, https://doi.org/10.1111/j.1574-695X.2007.00332.x]
- ↑ Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004 Aug;34(9):1001-27. doi: 10.1016/j.ijpara.2004.06.004. PMID: 15313128.
- ↑ Abdul Mannan Baig Primary Amoebic Meningoencephalitis: Neurochemotaxis and Neurotropic Preferences of Naegleria fowleriACS Chemical Neuroscience 2016 7 (8), 1026-1029 DOI: 10.1021/acschemneuro.6b00197
- ↑ [Francine Marciano-Cabral, Guy A. Cabral, The immune response to Naegleria fowleri amebae and pathogenesis of infection, FEMS Immunology & Medical Microbiology, Volume 51, Issue 2, November 2007, Pages 243–259, https://doi.org/10.1111/j.1574-695X.2007.00332.x]
- ↑ Travis W. Heggie Thomas Küpper Surviving Naegleria fowleri infections: A successful case report and novel therapeutic approach Travel Medicine and Infectious Disease Volume 16 2017 Pages 49-51 ISSN 1477-8939
Edited by [Cameron McCaleb], student of Joan Slonczewski for BIOL 116, 2024, Kenyon College.
