Bovine Rumen

Biorealm Niche: Bovine Rumen
Bonvines are subfamily of Bovidae family, and they utilize rumination (re-chewing food) as a mechanism of feeding. They have four stomach compartments that allow them to be successful in rumination. Of the four, rumen is special in that it contains billions of microbes that help the bovines to digest the food.
A simple look at the bovine rumen shows an incredible variety of organisms ranging from fungi, bacteria, archaea, protista, and viruses, namely bacteriophages. Their functions are to help in the breakdown of the various foods that would pass through the rumen, namely plant matter such as grasses, and its various difficult components.
The interesting thing about the microorganisms that live in the bovine rumen is that they are all somewhat dependant on each other for survival, and utilize the byproducts of one another for their own benefit.
There is currently much research involving the topic of ruminant animals and the microbes that inhabit the rumen. The conversion of rumen bacteria into efficient sources of energy and fuel is ongoing research that could potentially help put a stop to increasing fuel prices in the future.
Description of Niche
What is Bovine Rumen (and other parts of bovine stomach)?
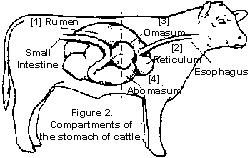
Bovines (of bovinae subfamily) are unique in a way that they have four stomach compartments to digest their food. Those compartments are rumen, reticulum, omasum, and abomasum. Of these, rumen is the largest compartment, and it can hold as much as 50 gallons of food and other ingested substances. The most important part about rumen is that it contains huge amount of different microbes, including bacteria, fungi, protozoa, and others. Reticulum, or "honeycomb," is the part responsible for rumination (or cud chewing) and trapping hard, indigestible substances like rocks, nails, or wires that may be ingested by accident while the bovine is grazing. If the reticulum gets punctured or injured from metal or other sharp objects, it can lead to traumatic reticuloperitonitis, or "hardware disease," and it is very important that this does not happen (1). Omasum, or "many-piles," is a leaf-like folds shaped compartment that acts as a gateway to the abomasum. Its main role is to send back large substances back to rumen and reticulum while allowing smaller, well-broken down substances to pass through into abomasum. Then, abomasum, also called "true stomach," is very similar to human stomach, as it is responsible for producing acids and enzymes to break down proteins, and it sends the chyme to small intestine (2).
In the rumen, there are billions of microbes living in a symbiotic manner. There are about 25 to 50 billion bacteria and 200 to 500 thousand protozoa. These microbes help the bovine to breakdown ingested food, while the bovine provides them with shelter and nutrients. Without them, bovines would have tough time digesting plant fibers and starches and producing useful fatty acids. Bovines can eat a wide range of feeds because they have many different kinds of microbes to help them digest. They eat and ruminate/regurgitate the incompletely chewed bolus back into the mouth so they can chew again. This process helps the microbes to have access to more finely chewed food in order to make microbial digestion easier. They can even eat urea because the microbes can use non-protein nitrogen to create amino acids. The microbes also produce other essential substances like vitamins B and C (2).
Although rumen-microbial system is a powerful factory for food consumption, it is also sensitive to sudden changes in feed-types. For example, if the bovine is accustomed to feeding on high levels of fiber and low levels of starch, then the rumen is equipped with more fiber-digesting microbes. Then, if the food content is suddenly changed to high starch and low fiber, the bovine would not be able to properly digest the feeds. This may lead to sudden decrease in pH, as the starch just sits in the rumen and ferments, and can cause harmful illness such as acidosis. Therefore, it is crucial to keep the nutrition of the bovines constant, especially for the domestic cows and oxen (2).
The bovines are also responsible for producing large amounts of methane gas, due to many methanogens like the strains of Methanobrevibacter, Methanomicrobium, Methanobacterium, and Methanosarcina living in the rumen. About 6% of what they eat gets lost as methane gas. This has been brought up in issues about global warming, and the effects of bovine methane in the environment are currently being researched (3).
Physical Conditions
Inside of the bovine rumen tends to have pH at around 6.5 to 7.2. Malnutrition, such as over-feeding of starch, can decrease pH level drastically and can be very harmful for the bovines. 20-35 gallons of saliva produced per day provides sodium bicarbonate to keep the pH consistent for the microbes to grow. Most of water in saliva is not wasted, but recycled in the body (2).
Since it is inside of bovine body cavity, the oxygen level is low or absent in the rumen, so it is an anaerobic condition. The absorption of the carbohydrates and volatile fatty acids is done by the epithelium of the rumen, which allows the ruminal juices to seep in between the cells. To increase the surface area for absorption, the ruminal mucosa contains numerous conical papillas (1).
The temperature of a healthy bovine tends to range from 37.8 °C to 40.0 °C, the core organs being around the high end. Temperature of the bovine depends mainly on body part and time of the day. For body part differential, the closer to the core, the higher the temperature becomes. Thus, temperature of the rumen is around 40.0 °C. Moreover, rumen contents tend to ferment at 40.0 °C, so this works as a central self-heating system. Temperature of the skin can be as much as 10 to 20 °C lower than the core temperature. Furthermore, time of the day makes difference in bovine body temperature. The temperature is usually cooler in the morning after resting and warmer in the night after long day of muscular activity. Recent studies have shown that hormonal interactions after giving birth can affect female cow’s body temperature as well. The range of healthy body temperature is fairly narrow, and thus it is very important to maintain that range in order to carry out normal metabolic and microbial functions (4).
These conditions show that the microbes in the rumen are obligate anaerobic mesophiles that only grow in non-extreme conditions.
Environment (Bovine Habitat)

The environment in which different bovine live can make a huge difference in the rumen function and physiology. The most well known example of a bovine for Americans is the domestic cow. Since these animals are domesticated, they are found in agriculture areas all over the world. Some are kept in tightly packed farms and others are able to graze across grasslands. These grasslands are not fertilized to produce any source of food for the cattle. Cattle that graze typically eat grass, stems, and other plant material (5). A cow’s rumen has a pretty standard pH of 5.8 to 6.2 and a temperature of 100 to 108°F. The pH is dependent on the number of the microbes found in the rumen which is determined by the type and amount of food being consumed from the environment. The acidic rumen along with the microbes living there help ferment and breakdown the cellulose in the grass. The environment also has an impact on the size of the rumen, which in a domestic cow is 60 to 80 liters. Since cattle eat a lot of grass, a large rumen is needed to accommodate a large volume of food (6).
Another specific example of a bovine is the wild yak. Wild yak live in areas of uplands such as hills, plains, and mountainous regions, all of which lack trees. They are generally found in the mountains regions of Southwest China, as well as Tibetan Plateau (7). Yaks tend to intake less food than other bovines, giving the yak a much smaller rumen. The smaller rumen gives the yak a lower outflow rate of rumen fluid than typical cattle. This rate is just 3.1 to 3.5 liters per hour. This outflow rate is dependent on many factors of the environment, some of these factors being the solidity of the food, air temperature, and size of the food particles. Yaks also produce more volitile fatty acids than other ruminates. These fatty acids can affect the fermenting capacity inside the rumen. This higher production of volitile fatty acids is directly related to the roughage on which yak feed (8).
Another type of bovine is the water buffalo, also known as the wild Asian buffalo. These buffalo are found where water is easily available, usually in low-lying grasslands, woodlands, or forests adjacent to a body of water (9). Water buffalo feed on a greater range of food than cattle. They tend to eat dry weeds along with aquatic plants, even diving up to 2 meters under the floodwaters just to graze. Just as the yak does, water buffalo make a higher amount of volatile fatty acids and produces it more quickly than does cattle. This is also due to the roughage available in the environment (10). As compared to cattle, water buffalo have a very similar pH, yet there is a higher population of bacteria inside the rumen. More specifically, water buffalo rumen containes more bacteria and fungus, but lower amounts of protozoa. Since water buffalo have a more diverse feed, they use a higher diversity of organisms in the rumen to digest the food (11).
The environment is a huge determinant on the physical properties of the rumen among different bovine species. The amount of nutrition a species consumes can determine the size of the rumen. The types of food available in the environment can change the pH as well as the microbes living in the rumen. Some species often have different rate of outflow coming out of the rumen. All these differences are determined by the environment in which the specific bovine lives.
Nutrition

When a bovine eats, billions of bacteria, protozoa, yeast, and molds in the rumen help the animal to be able to eat and digest the great amount of grasses (protozoa and bacteria doing most of the work). They all live in a symbiotic manner with the cow, helping to breakdown difficult substances while living in a suitable niche. These microbes can aid in the digestion of many foods such as hay, grass, grain, corn, and even urea. When microbes breakdown and digest plant fiber they produce volatile fatty acids which are then absorbed into the rumen, supplying about 60 to 80% of the cow’s energy. The microbes also produce essential amino acids from the proteins in which the cow consumes (2).
A proper nutrition is important for bovine heath, especially for domestic cows raised for their milk or beef. Inadequate or unbalanced nutrition can lead to many problems including poor growth, increased cases of illness, and lower rates of reproduction. As previously described, bovine nutrition consists mostly of grasses, weeds and plants, but proper nutrition is not that simple. For example, the domestic cow requires a strict diet to remain healthy and maintain a well-functioning rumen. Some rules that producers must abide by are that diets should contain no wore than 5% fat, fiber should be kept between 30 to 70% of daily intake, and cattle that graze in fields with high concentration of legumes must be supplied with ionophores or poloxolene. All these rules were created so to promote a health rumen (2).
Nutrition can even be manipulated to manage milk composition. By maximizing the function of the rumen through feeding, milk component percentages and production are also optimized. A producer of milk cows can increase the feed intake of his cattle and cause an increase in milk production. Producers can also manage other factors so to manipulate the protein and fat percentages of the milk. For example, by decreasing the amount of fiber or by increasing the nonfiber carbohydrates, milk fat is decrease and protein is increased. When fiber is rationed, rumen fermentation causes an increase in propionic acid, causing and decrease in the percentage of milk fat. Also, by decreasing the crude proteins intake, a producer can cause a decrease in milk protein. By adjusting the nutrition of the cow, rumen function can be manipulated so to increase or decrease the percentage of milk fat and protein (12).
Who lives there (Microbes in Rumen)?
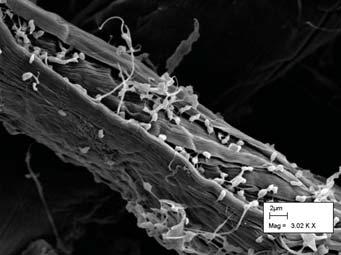
It should be of no surprise that an environment as complex as the bovine rumen would have a certain number of microorganisms that have adapted to the bovine rumen and taken it as its home. As cows and its cousins as ruminants are in fact "earth's dominant herbivores" (13), it should be of no surprise that some of the organisms would take advantage of their success and have actually created a sort of symbiotic relationship with the cows, helping them to digest their foods, especially ones containing cellulose, a material that composes plant cell walls, which they would not be able to do themselves.
A simple count by obtaining material from fistulated cows and diluting the material found that there were about 10^8-10^10 microorganisms per gram. This was the average number and found to be the range for about 94% of the trials, which included as variables the time of day, time between feedings, different cows, diets, and other factors. It was found that the most common microorganisms were gram-positive cocci and rods, which are in fact the most common types of bacteria (14). The bovine rumen is a niche that has a narrow pH range and is kept fairly stabilized, so that the bacteria in the rumen as well as the intake sacs for food and water are not too disturbed (15).
Cellulose is broken down by "communities of anaerobic microorganisms that exist in symbiotic association with the host" (16). While it may be surprising that a community of organisms is involved in the breakdown of the cell wall rather than a specific bacterium, it is discovered that there are not only bacterium, but also fungi and protozoa involved in this breakdown. Ruminococcus flavefaciens is a gram-positive bacterium that produces an enzyme complex anchoring to the cell wall and catalyzes various activities. Polyplastron multivesiculatum is a protozoan that contains individual enzymes that degrade the cell wall. Prevotella bryantil is a gram-negative bacterium that utilizes the soluble polysacharrides, namely xylans. This type of activity, polysacharride utilization, plays an important role in most complex gut niches.
The symbiotic relationship between the cow and its various bacterial friends comes into play when considering that the "bioconversion of lignocellulosic feeds into volative fatty acids" (15) that the microbes play the role of creates an energy source for the bovines. Specific breakdowns of the microorganisms are as follows: bacteria, at about 10^10-10^11 cells per milliliter, ciliate protozoa at about 10^4-10^6 per milliliter, anaerobic fungi at about 10^3-10^5 spores per milliliter and bacteriophages at about 10^8-10^9 per milliliter. It should be noted that many microbes cannot be cultured in labs, so the numbers may actually be higher in reality.
The products of each of these microbes create a sort of environment under which all of them survive. Some are dependant on each other and nourish each other, while other feeds can actually contain anti-nutritional variables which can actually suppress the growth of other microbial organisms. It is, however, the overall net result of the activity of the organisms which contributes to the utilizable source of energy and net gain for the cow (15).
The bovine rumen niche is described as being both "stable and at the same time dynamic." What this means is that he ecosystem within the cow's stomach serves its purpose very well, which is to convert the biological matters, namely grass, into a usable form of energy for the cows, such as volatile fatty acids. This system is also stable in the fact that despite the invasion of perhaps millions of foreign microbes daily through oral means, the cow is able to ward off the invaders and keep its function stable. However, the dynamic part of the ecosystem exists as the cow will often change its diet from day to day and week to week, but is able to adapt to any changes, whether they be small or large. This occurs due to the diversity of the microorganism community within the rumen, as well as the adaptability of the microorganisms (15).
Certain organisms such as Treponema bryantil, a saccharolytic spirochete, are associated with the breakdown of cellulose, although it is known not to be a utilizer of the material. It is known however that the material appeared after the cellulose hydrolysis by rumen bacteria. While cellulolytic bacteria themselves breakdown the plant cell walls into soluble sugars, it has been shown that the spirochete actually enhances the breakdown of cellulose (17).
Bacteria in the rumen generally feed on contents in the rumen which are composed of cellulose, lignin, starch, oil and protein. Through this decomposition as well as interaction with other microbial groups results in the production of the fatty acids that the cow depends on to live. In general, the bacteria in the bovine rumen tend to be gram-negative and are anaerobic. They grow at a slightly acidic pH level of 6.0 to 6.9, and at a temperature of a warm 39 degrees Celsius (15).
There are a few species of archeabacteria in the bovine rumen, which represent the methanogens. These organisms are present in very large numbers of the bovine rumen, but fluctuate depending on the type of diet given to the cow. The role of the methanogens generally is to scavenge hydrogens generated by rumen fermentation (15). The interest of the methanogens has greatly increased as a result of the find that about 6% of the energy that a cow consumes is then converted into methane. These methanogens are difficult to culture, however, as although they heavily populate the bovine rumen, they are difficult to grow within a lab setting (18).
Out of the various microorganisms in the bovine rumen, the fungi were actually mislabeled when they were first discovered as flagellate protozoa. The roles of the various fungi, such as Neocallimastix frontalis and other Sphaeromonas and Oikomonas, are generally to breakdown fibers. Experimentation has shown that the removal of such fungi from bovine stomachs has significantly reduced the amount of fibrous seed breakdown within the rumen (15).
Bacteriophages are in the bovine rumen in very large numbers, but they clearly aren't lysing bacteria at a rate that proves lethal to the cows. In fact, their role is most likely out of obligation so that the bacterial population doesn't become too high within the rumen, although this would only be convenient for cows with a consistant feeding schedule. The lysing of bacteria would have the effect of population control as well as the provision of amino acids for the cows to be able to utilize (15).
Methanogens
Many individuals attribute global warming to the ozone layer eroding away or the burning of fossil fuels. Many people do not realize that methane gas in our atmosphere is a huge contributor to global warming. More surprising is that about 15% of the methane
produced per year is from cattle (19). The organisms responsible for producing methane through bovine rumen are termed methanogens and do so in order to reduce the amount of carbon in the rumen system for fermentation (20). Bovine rumens that are host to a great number of methanogens generally come from cattle fed hay and alfalfa (21). There are two major divisions within the methanogens found in the bovine rumen. The Methanobrevibacter ruminatium and the Methanosphaera stadtmanae. The Methanobacteriaceae (contains the Methanosphaera stadtmanae) is the most common family found while the families Methanomicrobiaceae, Methanocorpusculaceae, and Methanospirillaceae are the second most populated methanogens found in the rumen (22).
Methanogens come from the domain Archaea and the kingdom of Eukarychaeota. They are obligate anaerobes whose main catabolic product is methane. They grow on a H2/CO2 gas mixture (23). They have high nitrate reductase activity (24). There are various strains of methanogens, but there a few specific strains that are more dominant in the bovine rumen. Methanobrevibacter are short bacterium that mainly utilizes H2 in the rumen. The strain Methanobrevibacter ruminatium or M1 requires the coenzyme M for growth and uses H2 as well CO2 as substrates. They tend to be gram positive and are classified as coccobacilli (20). They have a GC content of 33.68%. The genome integrates for DNA replication, phage integration, and the lysis. It has a low GC content at the end of the phage in order to protect against any foreign DNA. The bacteria has many surface proteins that help it communicate with other microbes that inhabit the rumen (20).
Other prominent genuses include Methanomicrobium, Methanobacterium, and Methanosarcina, which are the main methane utilizing bacterium.

A specific type of Methanomicrobium is Methanomicrobium mobile, which uses a growth factor called the mobile factor. These short microbes tend to be rod-shaped. Unlike many bacteria, they do not produce endospores. Their optimal growth level is 40 degrees Celsius and they prefer the pH level to be between 6.1 and 6.9 (25). These microbes tend to lyse after 2-4 days of life. They grow on acetate, isobutyrate, isovalerate, and 2-methylbutyrate. High concentration of acetate is particularly important for growth as is it for Methanobrevibacter ruminatium. Another similarity between Methanomicrobium mobile and Methanobrevibacter ruminatium is that they need branched chain fatty acids to survive (26).
Methanogens can be free-living but may also form symbiotic relationships with various protozoa.
Methanogens come in various shapes and differ in travel methods. The various strains of Methanobacterium formicicum exhibit these differences. For example, the BRM9 is rod shaped, is Gram positive, and is non-motile. On the other hand BRM16, which shares the rod-shape, differs in that it is gram negative and is motile. The both grow at about 38 degrees Celsius and at a pH level ranging from 6.5-7.0. CM1 is psuedosarcina shaped but is non-motile. It can grow best at about 40 degrees Celsius and at a pH of 6.5. The 16S rRNA gene is significant in exploring and researching methanogens (27).
Pectin-Fermenting Bacteria
Pectin is a naturally found in the cell walls of the non-woody parts of terrestrial plants. It helps bind the cells together and is found to be 10-20% of the total carbohydrate complex (majority if the rest is cellulose). Pectin-fermenting bacterium require a pH around 6.0 to thrive, although Bacteroides activity has been observed at a pH of 5.1. (4) A total of thirty-two strains of pectin-fermenting bacterium have been isolated from rumen fluid, of which 10 have been characterized and consolidated down to 3 individual strains: Lachnospira Multiparous, Butyrivbrio fibrisolvens, Bacteroides ruminicola. All strains are anaerobic and are generally 1.0-1.2 microns in diameter. (1)
Lachnospira Multiparous is gram positive and curved rod-shaped. Fermentation products are: formate, lactate, H, CO. Lachnospira is mobile in the rumen. (1)
Butyrivibrio fibrisolvens is gram positive and rod-shaped. Fermentation products are: butyrate, lactate, acetate, formate, H2 and CO2. Butyrivibrio are mobile in the rumen and also able to use cellulose as a food source along with pectin. (2)
Bacteroides ruminicola is gram negative and rod-shaped. Fermentation products are: formate, acetate and succinate. Bacteroides are not mobile. (3)
Other organisms
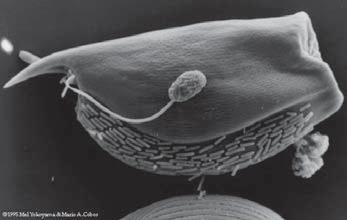
The rumen is a ripe place for anaerobic cell life. The conditions inside the rumen stay constant from bovine to bovine. It is not only a host to myriad of bacterium, but also viruses, fungi and protazoa. The other organisms inside the rumen both compete with and live in symbiosis with bacterium. The following picture shows bacterium in symbiosis with protozoa. (4)
However, it is also noted that some protozoa regularly graze on bacterium, as a bovine on grass. (4)
Are there any other non-microbes present?
Plants? Animals? Fungi? etc.
Do the microbes that are present interact with each other?
Describe any negative (competition) or positive (symbiosis) behavior
Do the microbes change their environment?
Do they alter pH, attach to surfaces, secrete anything, etc. etc.
Do the microbes carry out any metabolism that affects their environment?
Do they ferment sugars to produce acid, break down large molecules, fix nitrogen, etc. etc.
Current Research


It has been proven that an increase in stress which a young calf experiences during its early life correlates to a decrease in food intake. Such a decrease in food intake results in a low growth rate of microbes within the rumen lining of the animal. Research is currently being conducted through AgriLabs by performing tests to create new supplemental feed, known as ruminant stimulants, which encourages the growth of rumen microbes during high times of stress. These microbes are essential in breaking down the high fiber diet of ruminant animals by providing the enzymes necessary to break down the fiber. One supplement in particular, Amaferm, contains “all-natural, direct-fed microbial, plus essential vitamins, minerals, and trace minerals” [Ref#]. The goal of this supplement is to assist the microbe Aspergillus oryzae with fermentation so as to help degrade fiber and stimulate an increase in microbial populations within the rumen. AgriLab researchers assure that “Amaferm stimulates the growth rate of these major types of bacteria and anaerobic fungi. Amaferm is one of the most, if not the most, researched direct-fed microbial” [Ref#].
Further research on the topic of the microbe Aspergillus oryzae has found:
Through the Department of Animal Science and Industry at Kansas State University, studies were performed to further test the nature of microbe Aspergillus oryzae’s fermentation extract to see whether it aids in the fermentation process of food digestion and fiber breakdown of ruminant animals. For the study, Holstein steer were fed a diet consisting of alfalfa and hay, which is high in fiber. Samples of the rumen were then gathered for observations. Within a trial period of 96 hours, substantial degradation of fiber in the rumen was evident, as well as an increase in bacterial microbe activity. Inversely, no increase in fungal or protozoal activity was observed.
The experiment proved that Aspergillus oryzae fermentation extract does promote fiber degradation and specifically targets one subgroup of ruminal microbes, bacterial microbes. In accordance to AgriLab’s study of Amaferm, increase in bacteria in the rumen aids in the fermentation process. Fungal and protozoal microbe activity levels were not noticeably affected and thus are not vital to the fermentation process, in comparison to bacterial microbes. [Ref#]
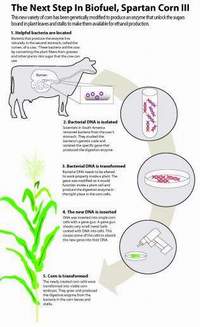
Researchers at Michigan State University’s crop and soil sciences division have been working on ways to convert cellular waste products from plants into an efficient source of fuel and energy through the study of cow bacteria. Within the rumen of the cow are microbes which contain fiber-degrading enzymes that help the cow break down and digest plant fibers such as cellulose, which cows are incapable of doing on their own. Researchers have developed a technique in which they isolate the gene that codes for the enzyme and then insert it into the DNA of corn plants. After allowing the corn plants to mature, it can be seen that they do obtain the same enzyme as the rumen bacteria. The current corn prototype has been given the title Spartan Corn III.
The enzyme currently only operates within the vacuole, the organelle which gathers and removes cellular waste from the cell. With help from the enzyme, this waste is broken down into simple sugars which can then be easily fermented into ethanol. With time, MSU hopes that the enzyme will be able to distribute throughout the entire plant, providing more areas of ethanol fermentation and ultimately, more sites where fuel can be produced. [Ref#]
References
Text
1. Wilson, Patrick D. "Bovine forestomachs and stomach". <http://www.vetmed.wsu.edu/van308/bovine.htm>. February 2004.
2. Hall, John B, Silver, Susan. "Nutrition and Feeding of the Cow-Calf Herd: Digestive System of the Cow". Virginia Cooperative Extension. <http://www.ext.vt.edu/pubs/beef/400-010/400-010.html#TOC>. June 2001.
3. Whitford, Marc F, Teather, Ronald M, Forster, Robert J. "Phylogenetic analysis of methanogens from the bovine rumen". PubMed Central. <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=32158>. May 2001.
4. Chen, Pei Jun. "Temperature of a Healthy Cow". Hypertextbook. <http://hypertextbook.com/facts/1998/PeiJunChen.shtml>. 1998.
5. Dewey, Tanya. “Bos Taurus”. Animal Diversity Web. http://animaldiversity.ummz.umich.edu/site/accounts/information/Bos_taurus.html . 2001.
6. Woodly, Bill, Metcalf, John A. “Understanding Rumen Function”. Dairy Digest. http://www.shurgain.com/pdf/Winter_2005_DairyDigest.pdf . Winter, 2005.
7. Massicot, Paul. “Animal Info- Wild Yak”. http://www.animalinfo.org/species/artiperi/bos_mutu.htm . March 2005.
8. Ruijun, Long. “14 Yak Nutrition- A Scientific Basis”. FAO Corporate Document Repository. http://www.fao.org/docrep/006/AD347E/ad347e0x.htm . 2003.
9. Massicot, Paul. “Animal Info- Wild Water Buffalo”. http://www.animalinfo.org/species/artiperi/bubaarne.htm . December 2004.
10. “The Water Buffalo: New Prospects For An Underutilized Animal”. http://www.dalnet.lib.mi.us/gsdl/cgi-bin/library?e=d-000-00---0demo--00-0-0-0prompt-10---4---Document---0-1l--1-en-50---20-help---001-011-1-0utfZz-8-0&a=d&c=demo&cl=CL2.1&d=HASH01d242dc3e08e1fdcf7343bb.8#HASH01d242dc3e08e1fdcf7343bb.8 . 1984.
11. Wanapat, M. “Swamp Buffalo Rumen Ecology and its Manipulation”. http://www.mekarn.org/procbuf/wanapat.htm . December 2001.
12. Looper, Michael R, Stokes, Sandra R, Waldner, Dan N, Jordan, Ellen R, “Managing Milk Composition”. Cooperative Extension Services College of Agriculture and Home Economics. http://www.cahe.nmsu.edu/pubs/_d/d-105.pdf . 2006
13. Hungate, R E. "The Rumen Microbial Ecosystem". Annual Review of Ecology and Systematics. <http://www.jstor.org/sici?sici=0066-4162(1975)6%3C39%3ATRME%3E2.0.CO%3B2-3>. 1975. Volume 6. p. 39-66.
14. Wilson, Marion K and Briggs, C. A. E. "The Normal Flora of the Bovine Rumen II Quantitative Bacteriological Studies". Journal of Applied Microbiology. <http://www3.interscience.wiley.com/journal/119888900/abstract?CRETRY=1&SRETRY=0>. 1955. Volume 18.2. p. 294-306.
15. Kamra, D N. "Rumen Microbial Ecosystem". Current Science. <http://www.ias.ac.in/currsci/jul102005/124.pdf>. July 2005. Volume 89.1. p. 123-135.
16. Flint, Harry J and Bayer, Edward A. "Plant Cell Wall Breakdown By Anaerobic Microorganisms from the Mammalian Digestive Tract". Incredible Anaerobes From Physiology to Genomics to Fuels. <http://www.annalsnyas.org/cgi/content/abstract/1125/1/280>. 2008. Volume 125. p. 280–288.
17. Stanton, T B and Canale-Parola E. "Treponema Bryantii, a Rumen Spirochete that Interacts with Cellulotytic Bacteria". Arch Microbiol. <http://www.ncbi.nlm.nih.gov/pubmed/7425785>. September 1980. Volume 127.2. p. 145-156.
18. Whitford, Marc F, et al. "Phylogenetic Analysis of Methanogens from the Bovine Rumen". BMC Microbiology. <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=32158>. 2001. Volume 1.5.
- "Remember the Rumen." Bovine Health Watch. 2007. <http://www.bovinehealthwatch.com/index.php?option=com_content&task=view&id=20&itemid=41>.
- Beharka, A.A., Nagaraja, T.G. "Effect of Aspergillus oryzae Fermentation Extract (Amaferm®) on In Vitro Fiber Degradation." Journal of Dairy Science. 1993. <http://www.dairy-science.org/cgi/content/abstract/76/3/812>.
- Swain, Heather C., ed. "Gut reaction: Cow stomach holds key to turning corn into biofuel." MSU Today 2008: 3. <http://news.msu.edu/magazine/media/08summer_research.pdf>.
Images
1. Weller, Keith. Image Number K5176-3. USDA Agriculture Research Service. <http://www.ars.usda.gov/is/graphics/photos/k5176-3.htm> 2006.
2. Hall, John B, Silver, Susan. "Nutrition and Feeding of the Cow-Calf Herd: Digestive System of the Cow". Virginia Cooperative Extension. <http://www.ext.vt.edu/pubs/beef/400-010/400-010.html#TOC>. June 2001.
3. McCowan, R.P., Cheng, K.J., Costerton, J.W. "Adherent bacterial populations on the bovine rumen wall: distribution patterns of adherent bacteria". PubMed Central. <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=291309>. January 1980.
4. "A-Z Animals". Arignar Anna Zoological Park. <http://www.aazoopark.gov.in/a-z.html>. January 2006.
5. Moss, Richard. "Dairy Replacement Heifers". Department of Primary Industries & Fisheries. <http://www2.dpi.qld.gov.au/dairy/6710.html>. May 2001.
8. USDA U.S Dairy Forage Research Center. "Fistula sample". <http://www.ars.usda.gov/Aboutus/docs.htm?docid=15329>. 2008.
9. Bauer, Scott. Image Number K9481-1. USDA Agricultural Research Service. <http://www.ars.usda.gov/is/graphics/photos/jul01/k9481-1.htm> 2006.
10. Moon, Mariella. "Spartan Corn III--Making Cellulosic Ethanol Production Viable." Good Clean Tech. <http://www.goodcleantech.com/2008/04/spartan_corn_iiimaking_cellulo.php>. April 2008.
Edited by students of Rachel Larsen David Becker , Jin Choi , Sarah Flores , Prema Hampapur , Edmond Ho , Adrianne Kurkciyan , Christopher Park , Brian Sun