Mycobacterium leprae in India
Introduction
Classification
Bacteria Actinobacteria Actinobacteridae Actinomycetales Corynebacterineae Mycobacteriaceae Mycobacterium leprae (1)
General
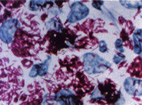
Mycobacterium leprae causes the chronic infectious disease called leprosy also known as Hansen's disease named after Gerhard Henrik Armauer Hansen (29 July 1841 – 12 February 1912) a Norwegian physician, who was the first to identify it in 1873 as the causative agent of leprosy (2). M. leprae is a gram-positive rod-shaped, acid-fast bacillus. The disease mainly affects the skin, the peripheral nerves, mucosa of the upper respiratory tract and also the eyes, apart from some other structures (3).
History
Based on historical evidence of skeletons and early written texts, leprosy is thought to have first originated from India (4)(13)(5). Leprosy in India dates as far back as the second millennium B.C. Recent findings of a male skeleton, after being tested for age, shows that the skeleton is from 2000 B.C. The skeleton shows symptoms of leprosy including degenerative joint disease and injury to the peripheral skeleton. This skeleton is the oldest evidence of leprosy in India which showed the earliest indication of human infection with Mycobacterium leprae in the world (4). From India, the disease was then spread to China, Egypt, and the Middle East, and later to Europe and the Americas through trade and war (5).
Olden India (the second millenium B.C.) categorized two types of leprosy: anaesthetic and tuberculated. Lepra anaesthetica is a more rare form of the two. It alters cutaneous nerves of body parts. Lepra tuberculosa is the general form of leprosy. It deteriorates the skin and tissues of the body (5). Symptoms described from olden India perfectly match what are the current symptoms of Kushtha (leprosy in Indian) today. The disease has been known to exist in India for, at the very minimum, three thousand years. About twenty persons out of ten thousand had this disease in 2000 B.C. Some cases of leprosy have extremities in terms of duration, how long the disease affects the patient. The shortest duration in a recorded case of leprosy has been one year, and the longest duration for a recorded case has been up to forty years (6).
Leprosy seemed to be especially prevalent in the district of Kumaun in the late 1800’s. In a statistical study, the disease was more prominent in the eastern side of the district rather than the western side. Research showed that there would not be an increase in leprosy of the population of Kumaun, as long as the disease was hereditary. Leprosy was received genetically and was not contagious, so it would not spread throughout Kumaun (6).
In a 1852 census, for every four males that had leprosy, only one female had leprosy. In the previous census before the 1852 census, the ratio for males to females with leprosy was almost ten to one. For some reason, the leprosy ratio decreased rapidly in 1852. However, with more recent studies, it seems that leprosy has been affecting females more than males. For ages 20-30, the percentage of males diseased is 20.0% whereas for females the percentage is 26.4% (6).
Description of Mycobacterium leprae
Genome
The complete sequence is 3,268,203 bp in length with a G+C content of 57.8%, and was generated from a combination of cosmid and 6-fold whole-genome shotgun sequencing. The start of the sequence is the first base of the dnaA gene, close to the origin of replication. There are 1,604 protein-coding genes and 1,116 pseudogenes. Both the sequence and annotation have been deposited in the public databases (NCBI) with the accession number AL450380 [1]. The Sanger Institute sequenced Mycobacterium leprae in collaboration with the laboratory of Stewart Cole at the Unit de Genetique Moleculaire Bacterienne, Institut Pasteu. Sequencing was funded by the Heiser Program for Research in Leprosy and Tuberculosis of The New York Community Trust, L'Association Raoul Follereau, The Wellcome Trust, ILEP, and the Institut Pasteur (7).
Transmission of Disease
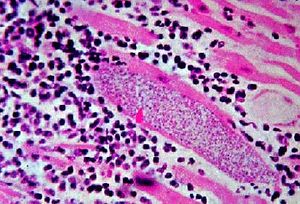
Traditionally, Mycobacterium leprae was known to infect and affect the skin, mainly through lining containing epithelial cells and also the peripheral nerves such as those contained in the central nervous system (8). However, there are recent research attempts to elaborate further into such findings to narrow down a specific mechanism of infection, which, to this day, is still unknown. Although a specific method proves lacking, there seems to be various hypotheses surrounding this greatly debatable topic.
Thus far, it has been postulated that Mycobacterium leprae may gain access to entry through two primary routes- the nasal mucosal membrane or through skin injuries. However, it has been shown that passage through the nasal mucosal membrane may be the primary route of infection. A study, focusing primarily on the mce1A gene in mce1 operon (mammalian cell entry) of Mycobacterium tuberculosis which proved to have a similar set of encoded proteins as Mycobacterium leprae; experiment was to be done in vitro, to observe the cell uptake activity of polystyrene latex beads which are layered with purified recombinant r proteins expressed by a specific locus within the mce1A gene (8). The results showed that the r-protein did promote uptake of the polystyrene latex beads into human nasal mucosal cells which concludes that the mce1A gene could mediate the entry of Mycobacterium leprae into respiratory mucosal tracts and may possibly be the main mode of transmission of such microbe. Additionally, endothelial cells may perhaps serve as a microbial reservoir, the cause of long-term infections. Unlike many other mycobacteriums, Mycobacterium leprae does not only survive in macrophages in vivo, it can also survive in nonmacrophage cells such as epithelial cells (as shown previously) and Schwann cells (9). Extensive research has revealed that destruction of such nonmacrophage cells is the primary manifestation of the disease, leprosy. It has been found that a Mycobacterium leprae gene encoding fibronectin (FN) binding protein is the main mechanism of transmission via epithelial and Schwann cells (9). The interaction between FAP-L (FN attachment protein) and FN is an important step in the pathogenesis of leprosy; FN acts as an oposinin (8).
Symptoms
When Mycobacterium leprae infects the body, the bacterium takes twenty seven hours to replicate due to the restricted intake of nutrients through the pores in their large, waxy walls. Because it multiplies very slowly, symptoms typically do not begin until three to five years later. This period between infection and symptoms is the "leprosy incubation period" which can also range from six months to several decades (10).
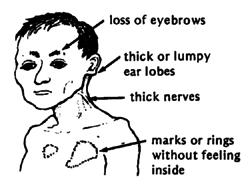
Leprosy affects primarily the skin and peripheral nerves which can cause muscle weakness resulting in deformities, inability to feel pain, and deterioration of senses. People unknowingly harm themselves, losing limbs. Distinguishing rashes and bumps often appear (11). Muscles become weak and skin becomes numb in areas controlled by the infected nerves. However, symptoms often range in type and severity and vary based on the form of leprosy:
In Tuberculoid Leprosy, a rash appears with flat, whitish areas on the skin . The bacteria damaging the underlying nerves cause the areas to be numb also (11).
In Lepromatous Leprosy, many small bumps or larger raised rashes of differing sizes and shape will develop. Lepromatous Leprosy has more symptoms of numbness and muscle weakness and has and increased chance of infection of the kidneys, nose, eyes, feet and testes than Tuberculoid leprosy. This can result in kidneys malfunction and failure, chronic stuffy nose and nosebleeds, glaucoma or blindness, foot sores, infertility and erectile dysfunction (11).
Borderline leprosy includes features of both tuberculoid and lepromatous leprosy (11).
If untreated, the immune system may produce inflammatory reactions, which can cause fever and inflammation of the skin and peripheral nerves. The skin may swell and become red and painful and the bumps may form open sores (11).
Why Leprosy is a Problem in India
In ancient India, there were rules against contact with those affected by leprosy and punished those who married into their families. The cultural response to leprosy often included loss of social position and expulsion/seclusion, even of kings, from the community due to people’s great fear of contagion, its visible disfiguring disability, association with sin, and its incurability at the time. With increasing pressure from the public who were afraid of the contagious infection, the government enacted the Leprosy Act of 1898 (12). This law allowed forcible confinement, segregation by gender to prevent reproduction resulting in thousands of leper colonies which still exists today even after the act was repealed in 1983. However, in a population of more than a billion people, up to 100,000 people suffering from leprosy still remain (13).
Even with curable drug treatment, leprosy is still a major problem in India, with over 50% of all leprosy patients located in India. The infection has thrived in India due to its poverty-induced, unclean, confined living conditions. Leprosy was often found in those who were undernourished and malnutritioned (5). Lacking money and knowledge, many still do not seek for help. Leprosy control and elimination in India still faces many challenges also due to cultural aspects of leprosy. Many Indians choose to seek private nonallopathic (traditional) practioners who outnumber the allopaths in India. These private nonallopathic practioners continue to use botanicals and agents like chaulmoogra oil from ancient India’s practices which does not cure leprosy, only healing the wounds making the patients still contagious (13). Changes in knowledge, attitudes, and practices needs to take place in order to eliminate leprosy from India.
Leprosy in Present Day India
Background Information
In the past, India had the highest percentage in contributing to prevalence and newly detected cases of leprosy worldwide (64% of the prevalence of leprosy and 78% of the new cases detected worldwide) (14). As of July 2006, 23 States and four Union Territories in India were proclaimed to have met the goal of leprosy elimination (prevalence <1 case per 10,000 population) as a public health problem having prevalence rate of 0.88 per 10,000 population; however, six states and two Union Territories are still working on achieving this goal (15). The trend of annual declining rate of prevalence and newly detected cases continues. According to the World Health Organization (WHO) report of India in 2008, the prevalence was 86,331, number of new cases detected was 134,184, number of new cases with multibacillary (MB) disease was 64,949, number of female leprosy cases was 47,188, number of new cases among children was 13,610, number of new cases with grade 2 disability was 3,763, and the number of relapses was 325 (16); all of these reported cases have reduced from previous years. This constant decrease was mostly due to programs such as National Leprosy Elimination Program (NLEP), Modified Leprosy Elimination Campaign (MLEC), and two research and training institutes Chingelput and JALMA, Agra that were founded to prevent and treat the spread of leprosy.
Prevention
Early detection is crucial for prevention of leprosy; therefore, NLEP was established in 1983 and MLEC in 1998. Through NLEP, special hospitals providing prevention and treatment for lepers were set up throughout India particularly in states that were faced with the highest leprosy cases (14). Before NLEP was established, there were approximately 4,000,000 cases and the prevalence rate was >50 cases/10,000 population (17). As with most diseases, there are patients who are not recorded in statistics. In order to reduce the amount of undetected cases of leprosy, MLEC was created, which employed staff and community volunteers to go house to house in search of individuals who may be at risk of developing leprosy (15). Besides these two programs, two research and training institutes Chingelput and JALMA, Agra were set up in 1972 “for further scientific and advance studies on leprosy (15)." This research will help with further understanding of leprosy which assists in providing better ways of minimizing the prevalence of leprosy. Through these programs, dermatologists and epidemiologists can work together to detect whether a patient has leprosy. A patient sees the dermatologist to determine whether there is a chance that they may have leprosy by using tests such as the PCR (polymerase chain reaction), ELISA (enzyme-linked immunosorbent assay) and other serological tests such as the lateral flow (ML-Flow) as technologies employed which helps detect leprosy (18). If a patient has leprosy, they are immediately put under multi-drug therapy in order to eradicate the disease.
Treatment
The treatment of leprosy has evolved through the years from not having an active system to the first breakthrough which occurred in the 1940s with the development of the drug dapsone, which arrested the disease. But the duration of the treatment of leprosy was many years, even a lifetime, making it difficult for patients to follow. In the 1960s, Mycobacterium leprae started to develop resistance to dapsone, the world’s only known anti-leprosy drug at that time but soon rifampicin and clofazimine, were discovered in the early 1960s. In 1981, the WHO Study Group recommended multidrug therapy (MDT) which consists of three drugs: dapsone, rifampicin and clofazimine (15). Though the three drugs was the main combination, there are different combinations of multidrug therapy such as antimicrobial agents such as rifampicin (RIF), rifapentine (RFP), moxifloxacin (MXF) and R207910 (a diarylquinoline).(18) While the multi-drug therapy alone takes 12-24 months to fully rid the body of leprosy, including a vaccine with the multi-drug therapy reduces the duration of treatment by at least six months in most cases (19). Even though multidrug therapy can rid the body of leprosy, there are known incidents where patients had a relapse period which allowed the disease to come back into the body years after the patient was free of leprosy. The WHO has estimated a risk of relapse of 0.77% for MB and 1.07% for PB patients 9 years after stopping MDT. Various other studies using person-years of observation estimate relapse rates varying from 0.65 to 3.0% for PB and 0.02 to 0.8% for MB leprosy (20). In order to be prepared for the worse, patients must understand that nerve damage is one of the results of leprosy which may occur before diagnosis of the disease, during treatment or after treatment (21). The purpose of controlling leprosy is to reduce the rate and severity of disabilities. Therefore, the main objectives in leprosy management are the early diagnosis and treatment, followed by an early recognition of nerve damage and effective intervention (18). Patients need to be taught how to take care of their hands and feet by inspecting their limbs daily. If a deformity occurs, patients must be provided with specialist footwear to prevent ulceration (21). With these steps of prevention, people infected this disease can be treated by physicians to completely eradicate their body of leprosy.
Future Strategies in Dealing with Leprosy
With constant effort to decline prevalence and detection rate of leprosy, WHO proposed strategies that will maintain and contribute in decreasing the disease burden. These strategies include: strengthening the incorporation of leprosy services into general health system and major support from “regional network” for ensuring early detection of new cases and immediate treatment with MDT and “collaboration with partners and community-based rehabilitation activities” will be acquainted to leprosy victims and their families (16). Also supporting IEC/advocacy activities to raise awareness and diminish the stigma of leprosy, and “promoting research aimed at improved tools, better approaches and cost-effective implementation (15).” This type of research on Mycobacterium leprae will be difficult to achieve because M. leprae is hard to study. M. leprae cannot be cultivated on artificial media, and the only established means to quantify viability of M. leprae has been by its relative growth in the foot pads of conventional mice (MFP) (22). The MFP method is technically difficult and requires several months to yield results; therefore, in order to have a successful research results, more effective methods are needed. If these strategies were followed, there is an expectancy decline of “the physical, mental and socioeconomic burden caused by the disease (16).”
References
1. "Classification." The DSMZ - Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (German Collection of Microorganisms and Cell Cultures). 2000. <http://www.bacterio.cict.fr/classificationmr.html#Mycobacterium>.
2. Irgens LM. "[The discovery of the leprosy bacillus]." Mar. 2002. <http://www.ncbi.nlm.nih.gov/pubmed/11998735>.
3. "Mycobacterium leprae is the bacterium that causes leprosy." Nature 409. 2001. <http://www.ebi.ac.uk/2can/genomes/bacteria/Mycobacterium_leprae.html>.
4. Robbins, Gwen. "PLoS ONE: Ancient Skeletal Evidence for Leprosy in India (2000 B.C.)." PLoS ONE : accelerating the publication of peer-reviewed science. 27 May 2009. <http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0005669>.
5. "Oldest Evidence Of Leprosy Found In India." Science Daily: News & Articles in Science, Health, Environment & Technology. <http://www.sciencedaily.com/releases/2009/05/090526202805.htm>.
6. Cunningham, David D. "Leprosy in India: a report." Google Books. Calcutta, 1877. 7-71. <http://books.google.com/books?id=bDMAAAAAQAAJ&dq=leprosy+in+india&printsec=frontcover&source=bl&ots=BAqiQpUlN2&sig=uUKTY8KP9Elqiz1hkaHg4CmVDWM&hl=en&ei=YpSTSvDjGpCgsgPLjti-Dw&sa=X&oi=book_result&ct=result&resnum=9#v=onepage&q=&f=false>.
7. S.T. Cole, K. Eiglmeier, J. Parkhill, K.D. James, N.R. Thomson, P.R. Wheeler, N. Honore, T. Ganier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R.M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M.A. Quail, M-A. Rajandream, K.M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J.R. Woodward and B.G. Barrell. "Massive gene decay in the leprosy bacillus" Nature 409, 1007-1011 (2001).
8. Sato N., Fujimura T., Masuzawa M., Yogi Y., Matsuoka M., Kanoh M., Riley L.W., Katsuoka K. "Recombinant Mycobacterium leprae protein associated with entry into mammalian cells of respiratory and skin components". Journal Dermatological Science. 2007 May;46(2)pg 101-10.
9. L. A. Noon, A. C. Lloyd. "Hijacking the ERK signaling pathway:Mycobacterium leprae shuns MEK to drive the proliferation of infected Schwann cells". Science STKE 2005, pg 52.
10. Schoenstadt, Arthur. "Leprosy Symptoms." Diseases Home Page. Clinaero, Inc., 15 Aug. 2008. <http://diseases.emedtv.com/leprosy/leprosy-symptoms.html>.
11. Nardell, Edward A. "Leprosy: Infections: Merck Manual Home Edition." Merck manuals online medical library. Merck & Co. Inc., Oct. 2008. <http://www.merck.com/mmhe/sec17/ch194/ch194a.html>.
12. Primm, T. P., C. A. Lucero, and J. O. Falkinham III. 2004. "Health impacts of environmental mycobacteria". Clin. Microbiol. Rev. 17:98-106.
13. Jacob, Jesse T., and Carlos Franco-Paredes. "The Stigmatization of Leprosy in India and Its Impact on Future Approaches to Elimination and Control." Jan. 2008; 2(1): e113.
14. Lockwood D, Suneetha, "Leprosy: too complex a disease for a simple elimination paradigm". World Health Organization 2005. pg 230-235.
15. "Leprosy in India". World Health Organization, 22 December 2006, Accessed August 25, 2008. <http://www.searo.who.int/EN/Section10/Section20/Section1999.htm>
16. Weekly Epidemiological Record. Vol. 84. No 28. 2009 August 14. < http://www.who.int/wer/2009/wer8433.pdf>
17. D. M. Scollard, L. B. Adams, T. P. Gillis, J. L. Krahenbuhl, R. W. Truman, D. L. Williams. "The Continuing Challenges of Leprosy". CLINICAL MICROBIOLOGY REVIEWS, Apr. 2006, p. 338–381.
18. Bernardes, Goulart, "Leprosy: diagnostic and control challenges for a worldwide disease". Springer-Verlag 2000. pg 269-290.
19. Mudur "India approves leprosy vaccine". British Medical Journal 1999. Volume 316 pg 414.
20. Kaimal S, Thappa "Relapse in leprosy". Indian Journal of Dermatology, Venereology and Leprology. 2009. Vol. 75, Issue.2; pg 126-134.
21. Lockwood D, Kumar, "Treatment of Leprosy: The evidence base for newer drug combinations and shorter regimens is weak". British Medical Journal 2004. Volume 328: pg 1447-1448.
22. Truman RW, Krahenbuhl JL. "Viable M. leprae as a research reagent". Int J
Lepr Other Mycobact Dis. 2001.
23. "Hansen's Disease Treatment". Kalaupaupa National Historical Park. 25 July 2006. <http://www.nps.gov/kala/historyculture/hansens2.htm>.
24. "National Leprosy Eradication Program." Government of Karala: Modern Medicine and Health Service. 2005. <http://www.healthkerala.gov.in/modern/jsp/Leprosy.jsp>
Edited by [Katherine Tang, Victor Tran, Natalie Nguyen, Julia Chu, Millie (Mei) Liu, Jason Wang], students of Rachel Larsen Summer 2009