Marivirga tractuosa
A Microbial Biorealm page on the genus Marivirga tractuosa
Classification
Higher order taxa
Domain: Bacteria
Phylum: Bacteroidetes
Class: Sphingobacteria
Order: Sphingobacteriales
Family: Flammeovirgaceae
Genus: Marivirga
Species
Marivirga tractuosa Type strain: H-43
Description and significance
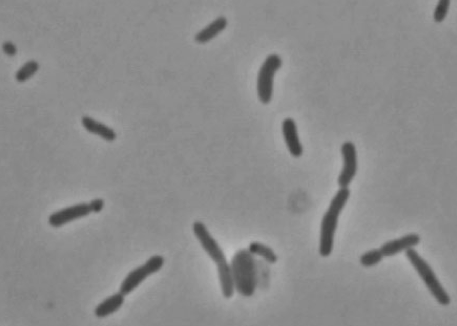
Marivirga tractuosa a rod-shaped,gram-negative, pigmented, non-spore forming bacterium. It is a mesophile with optimum growth temperatures from 28-32ºC. It is important because it is resistant to some antibiotics and it has an interesting motility. This microbe has a gliding motility, meaning that it moves by itself without the help of flagella or any external forces. The antibiotics it is resistant to include: gentamicin, kanamycin, neomycin, polymixin and streptomycin.
Genome structure
Marivirga tractousa chromosome is 4,511,574 base pairs long, and the chromosomes are circular. They have a 4916 sequence length, and they have 4516490 nucleotides. It has 3757 protein genes and 49 RNA genes. Its DNA coding region is at 4,029,412 base pair, and its DNA G+C content is 1,604,111 base pairs. Its total number of genes is 3,857. It has 2 rRNA operons, and 51 Psuedo genes.
Cell and colony structure
Marivirga tractuosa are long, slender, flexible rods 10-50 µm in length, 0.4-0.5 µm in width Colonies are circular, shiny and 2-4 mm in diameter after 72 hours of incubation on marine agar (which is a gelling agent from seaweed). They are usually dark-orange in color but whitish or yellow-pigmented are also sometimes present.
Metabolism
Oceanibulbus indolifex is an obligate aerobe, non-fermentative bacterium that requires oxygen to grow. The bacterium is a heterotroph that utilizes D-glucose, pyruvate, DL- lactate, serine, ornithine, alanine, asparagine, L-aspartate, L-glutamate, L-proline, succinate, mannitol, adipate, malate, citrate and glycerol as its carbon source and external electron donor reductant sources. Oceanibulbus indolifex contains Q10 (uibiquinone 10) as its dominant respiratory quinone in the electron transport chain to aid in ATP synthesis. Q10 is specific to members of Alphaproteobacteria class. Oceanibulbus indolifex does not hydrolyze gelatin, starch, urea, or aesculin. The bacterium tested slightly positive for the presence of cytochrome oxidase a transmembrane protein complex in the electron transport chain that transfers electrons to oxygen and translocates four protons per electron to help create a gradient for ATP synthesis. Oceanibulbus indolifex does not reduce nitrate to nitrite. The bacterium produces indole, cyclic dipeptides, and thryptanthrin.
Ecology
Oceanibulbus indolifex has optimum growth from 25-30°C but can grow in a range all the way down to 8°C. The bacterium’s optimum growth occurs at a pH of 7 but it can tolerate a range from 7-9. The optimum salts concentration for growth is 3-5% and the halotolerance range is from 1-10%. Oceanibulbus indolifex does not grow in medium lacking salts or in medium containing only sodium chloride.
Pathology
Oceanibulbus indolifex has no known pathogenic effects on humans. The bacterium does have genes coding for β-lactamases indicating resistance to β-lactam antibiotics like penicillins, cephamycins, and carbapenems. Oceanibulbus indolifex is susceptible to aminoglycosides, antibiotics derived from bacteria of the genus Streptomyces that interfere with bacterial ribosome function.
References
[[2]Wagner-Do ̈bler, I., Rheims, H., Felske, A., El-Ghezal, A., Flade-Schro ̈der, D., Laatsch, H., Lang, S., Pukall, R., Tindall, B., 2004. Oceanibulbus indolifex gen. nov., sp. nov., a North Sea alphaproteobacterium that produces bioactive metabolites. International Journal of Systematic and Evolutionary Microbiology doi: 10.1099/ijs.0.02850-0]
[[3]Piekarski, T., Buchholz, I., Drepper, T., Schobert, M., Wagner-Doebler, I., Tielen, P., Dieter Jahn, D., 2009. Genetic tools for the investigation of Roseobacter clade bacteria. BioMedCentral Microbiology doi:10.1186/1471-2180-9-265]
[[4]Tang, K., Huang, H., Jiao, N., Wu, C., 2010. Phylogenomic Analysis of Marine Roseobacters. PLoS ONE 5(7): e11604. doi:10.1371/journal.pone.0011604]
[[5]Thiel, V., Brinkhoff, T., Dickschat, J., Wickel, S., Grunenberg, J., Simonb, M., Schulz, S., 2009. Identification and biosynthesis of tropone derivatives and sulfur volatiles produced by bacteria of the marine Roseobacter clade. Organic and Biomolecular Chemistry DOI: 10.1039/B909133E]
Edited by Ariel Kaplan of Dr. Lisa R. Moore, University of Southern Maine, Department of Biological Sciences, http://www.usm.maine.edu/bio
