Marinobacter hydrocarbonoclasticus
Classification
Lineage: Bacteria; Proteobacteria; Gammaproteobacteria; Alteromonadales; Alteromonadaceae; Marinobacter; Marinobacter hydrocarbonoclasticus Species: Marinobacter hydrocarbonoclasticus
Bacterial Characteristics
Marinobacter hydrocarbonoclasticus was first isolated near a petroleum refinery in the Mediterranean Sea. It is a gram-negative, rod-shaped, marine bacterium and is not capable of sporulation. On average, they are between 0.3-0.6 µm in diameter and 2-3 µm long. [1] The cells have an unsheathed polar flagellum allowing them to be motile in the marine environment. They are also extremely halotolerance and somewhat halophilic [2]. They can grow in an environment with a NaCl concentration range of 0.08 M - 3.5 M, with an optimal NaCl concentration of 0.6 M. [1] The bacteria usually grow and live in an environment with a temperature of 10 - 45˚C and a pH of 6 – 9.5. [1] The NaCl content is important for M. hydrocarbonoclasticus , for the NaCl concentration has the ability to affect both shape and flagellation of these cells. [1] The species is able to degrade different kinds of hydrocarbons and use them as sole carbon and energy sources. Overall, M. hydrocarbonoclasticus has the ability to inhabit and grow in a wide range of environments in the marine ecosystem.
Genome Structure and Phylogeny
Marinobacter hydrocarbonoclasticus has a total genome size of approximately 4 Mb with a G + C-content of 57.4%. [1] The overall genome size of the bacteria ranges from 4.3 – 4.9 Mb , which is smaller than the other Marinobacter genomes such as Marinobacter adhaerens . [3] The genome is composed of 3807 protein coding regions (~91% of the genome). [4] M. hydrocarbonoclasticus has the ability to form biofilms, which requires three groups of polysaccharide biosynthesis genes, four pilus genes clusters, and several housekeeping (lap ) genes. [4]
Furthermore, an analysis of 16S rRNA sequence indicates that M. hydrocarbonoclasticus belongs to the gamma-type Proteobacteria and is distinctive from bacteria with similar morphological and biochemical properties. The species is closely related to Marinobacter sp. strain CAB and Marinobacter aquaeolei , in which the 16S rDNA analysis indicated that M. hydrocarbonoclasticus is 99.8% similar to Marinobacter sp. strain CAB and is 99.4% similar to Marinobacter aquaeolei . [5] This demonstrates that there are other Marinobacter species that are extremely similar to M. hydrocarbonoclasticus on a DNA level.
Metabolism
Marinobacter hydrocarbonoclasticus exhibits catalase, oxidase, tweenase, and lecithinase activities, which enable the bacterium to degrade hydrophobic substances under an aerobic environment. [1] The cell is able to degrade both cyclic and non-cyclic alkanes. Since petroleum hydrocarbons are often non-cyclic alkanes, the species is able to degrade and use them as both carbon and energy sources. For example, it has significant hydrocarbonoclastic activities on hydrocarbons chains such as hexadecane (C16H34), eicosane (C20H42), pristane (C19H40), and others for energy and carbon usages. [6] Therefore, M. hydrocarbonoclasticus is a potential agent for bioremediation. It is important to highlight that the bacterium does not contain cytochrome P450, which is a terminal hydroxylase that is important for the breakdown of stable, aromatic rings in many petroleum hydrocarbons. [7] However, the species has the ability to anaerobically breakdown aromatic compounds, which makes the organism unique from many other hydrocarbon degraders.
Furthermore, Marinobacter hydrocarbonoclasticus bacteria are not restricted to hydrocarbons for energy and carbon sources. The cells can use nitrate (NO3-) or nitrite (NO2-) as the terminal electron acceptor to form gas product such as N2O, by consuming other organic substances such as citrate, acetate, or succinate as sources of carbon and energy. [1] Therefore, they are considered to be denitrifiers that could also grow under an anaerobic environment.
Petrobactin Siderophore
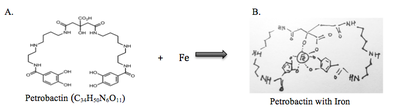
Iron is crucial for life as it is required DNA synthesis and cellular respiration. Soluble Fe(III) is very low and limited in concentrations in a marine environment (approx. 0.02−1 nM). [8] M. hydrocarbonoclasticus overcomes this problem by producing and secreting a siderophore compound called petrobactin. Petrobactin forms a complex with Fe(III) by providing the inorganic compound with six donor groups. [9] The six groups greatly increase the binding interaction between the organic and inorganic compounds. The solubilized iron is then absorbed by the bacteria and used as a source of nutrient.
The compound is also photolabile in its ferrated form, in which a light-mediated decarboxylation reaction results in a reduction of Fe(III) to Fe(II) when petrobactin is exposed to sunlight. [9] This indicates that too much light could inhibit the bacterial growth due to the lack of usable iron. Petrobactin have also shown to have an influence on the growth of M. hydrocarbonoclasticus . There is a direct correlation between the concentration of siderophore in the medium and the stimulating activity for bacterial growth. [10] However, the cell tightly regulates the uptake of iron. [11] As a result, additional siderophore produces an insignificant bacterial growth response.
Significance
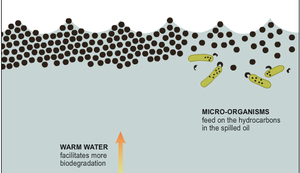
Petroleum hydrocarbons are potential pollutants that could cause serious damage to marine ecosystems. These compounds, mainly composed of non-cyclic alkane chains, are hydrophobic and recalcitrant by nature, making them extremely difficult to remove. Marinobacter hydrocarbonoclasticus , along with other species of Marinobacter, plays an important role in bioremediation. M. hydrocarbonoclasticus bacteria are specialists in degrading hydrocarbons, especially n-alkanes. This distinctive ability allows the cells to be an ideal organism for treating recalcitrant pollutants.
Marinobacter hydrocarbonoclasticus also can assist in the technological advancement of bioremediation by serving as an ideal model for studying hydrocarbon biodegradation, as it grows well in laboratory conditions and is specialized bio-degraders. For example, Hamdan and Fuller (2011) used a chemical dispersant, COREXIT® EC9500A, to study its effect on the bacteria. [13] They discovered that the chemical inhibited the proper functioning of hydrocarbon-degrading bacteria and could decrease bioremediation capacity. [13] Thus, researching Marinobacter hydrocarbonoclasticus aids in the bioremediation development by providing scientists with more knowledge regarding hydrocarbon-degrading bacteria.
References
(1) Gauthier, M. J., B. Lafay, R. Christen, L. Fernandez, M. Acquaviva, P. Bonin, and J.-C. Bertrand. “Marinobacter hydrocarbonoclasticus gen. nov., sp. Nov., a new, extremely halotolerant, hydrocarbon degrading marine bacterium.” Int. J. Syst. Bacteriol., 1992, DOI: 10.1099/00207713-42-4-568.
(2) Larsen, H. “Halophilic and halotolerant microorganisms: an overview and historical perspectives.” FEMS. Microbiol. Rev., 1986, DOI: 10.1111/j.1574-6968.1986.tb01835.x.
(3) Gärdes, A., Kaeppel, E., Shehzad, A., Seebah, S., Teeling, H., Yarza, P., Glöckner, F.O., Grossart, H.P., and Ullrich, M.S. “Complete genome sequence of Marinobacter adhaerens type strain (HP15), a diatom-interacting marine microorganism.” Stand. Genomic Sci., 2010, DOI: 10.4056/sigs.922139.
(4) Grimaud, R., Ghiglione, J.F., Cagnon, C., Lauga, B., Vaysse, P.J., Rodriguez-Blanco, A., Mangenot, S., Cruveiller, S., Barbe, V., Duran, R., Wu, L.F., Talla, E., Bonin, P., and Michotey, V. “Genome Sequence of the Marine Bacterium Marinobacter hydrocarbonoclasticus SP17, Which Forms Biofilms on Hydrophobic Organic Compounds.” J Bacteriol., 2012, DOI:10.1128/JB.00500-12.
(5) Nguyen, B.H., Denner, E.B.M., Dang, T.C.H., Wanner, G., and Stan-Lotter, H. “Marinobacter aquaeolei sp. nov., a halophilic bacterium isolated from a Vietnamese oil-producing well.” Int. J. Syst. Bacteriol., 1999, DOI: 10.1099/00207713-49-2-367.
(6) Al-Mallah, M., Goutx, M., Mille, G., and Bertrand, J.C. “Production of emulsifying agents during growth of a marine Alteromonas in seawater with eicosane as carbon source, a solid hydrocarbon.” Oil Chem. Pollut., 1990, DOI: 10.1016/S0269-8579(05)80005-X.
(7) Rehm, H.J., and Reiff, I. “Mechanisms and occurrence of microbial oxidation of long-chain alkanes.” Adv. Biochem. Eng., 1981, DOI: 10.1007/3-540-10464-X_18.
(8) Hickford, S.J.H., Küpper, F.C., Zhang, G., Carrano, C.J., Blunt, J.W., and Butler, A. “Petrobactin Sulfonate, a New Siderophore Produced by the Marine Bacterium Marinobacter hydrocarbonoclasticus.” J. Nat. Prod., 2004, DOI: 10.1021/np049823i.
(9) Raymond, J., Bergerona, R.J., Huanga, G., Smitha, R. E., Bhartia, N., McManisa, J.S., Alison, B. “Total synthesis and structure revision of petrobactin.” ChemInform, 2003, DOI: 10.1016/S0040-4020(03)00103-0.
(10) Gardner, R.A., Kinkade, R., Wang, C., and Phanstiel, O. “Total Synthesis of Petrobactin and Its Homologues as Potential Growth Stimuli for Marinobacter hydrocarbonoclasticus, an Oil-Degrading Bacteria.” J. Org. Chem., 2004, DOI: 10.1021/jo049803l.
(11) Schalinske, K.L., Blemings,K.P., Steffen, D.W., Chen, O.S., Eisenstein, R.S. “Iron regulatory protein 1 is not required for the modulation of ferritin and transferrin receptor expression by iron in a murine pro-B lymphocyte cell line.” Proc. Natl. Acad. Sci. U.S.A., 1997, DOI: 10.1073/pnas.94.20.10681.
(12) McGenity, T.J., Folwell, B.D., McKew, B.A., and Gbemisola-O-Sanni, G.S. “Marine crude-oil biodegradation: a central role for interspecies interactions.” Aquat. Biosyst., 2012, DOI: 10.1186/2046-9063-8-10.
(13) Hamdan, L. J. and P. A. Fulmer. “Effects of COREXIT® EC9500A on bacteria from a beach oiled by the Deepwater Horizon spill.” Aquat. Microb. Ecol., 2011, DOI: 10.3354/ame01482.
(14) “Anthrax bacteria’s biosynthesis has potential for the development of therapeutic treatments.” http://www.lanl.gov/orgs/b/b8/b8_highlight_2.shtml
(15) Barnes and Nobel. “Marinobacter Hydrocarbonoclasticus.” http://www.barnesandnoble.com/w/marinobacter-hydrocarbonoclasticus-lambert-m-surhone/1104974460.
(16) Kuang, k. "Infographic of the Day: The Physics of Oil Spills." Fast Company, 2010. http://www.fastcompany.com/1659822/infographic-day-physics-oil-spills.