Environmentally essential bacteria: Geothrix fermentans
Introduction
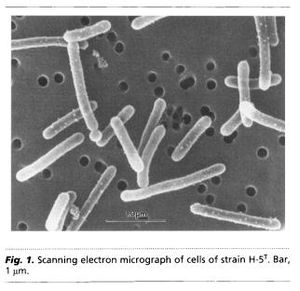
Geothrix fermentans are non-motile rod-shaped bacteria. Due to the Greek origin of its name “Geo” meaning earth and “thrix” pertaining to hair, G. fermentans are sometimes described as hair like organisms from the earth. They can occur either singly or in chain links. Their cell size is around 0.1 µm in diameter and between 2-3 µm in length (Figure 1.) (Coates et al., 1999). They are gram-negative bacteria that can be grown on and identified by MacConkey agar plates as they will appear pink in color due to a thinner peptidoglycan cell wall than in gram-positive bacteria. G. fermentans do not produce spores during their reproductive cycle. The optimal growth temperature is at 35°C and there has been no indication of growth below 25°C. Geothrix fermentans were discovered in 1999 at Hanahan, SC in the United States. By using 16S rDNA, we have determined that the closest identifiable bacterial relative of G. fermentans is Holophaga foetida with a 94.3% sequence identity (Slonczewski and Foster., 2010).
G. fermentans are commonly associated with aquatic sediments such as aquifers because they are anaerobic bacterium and aquifers provide an ideal environment for them to not only survive but flourish. G.fermentans are a member of the acidobacterium phylum a recently devised phylum that includes several bacteria that serve an important role as contributors to many different kinds of environmental niches. Other acidobacterium similar to Geothrix fermentans include Halophaga foetida, Bryobacter aggregatus, and Acanthopleuribacter pedis. G.Fermentans are physiologically associated with Geobacteraceae because they both are strict anaerobes that conserve energy to support growth by oxidizing organic acids into CO₂ by using Fe (III) oxides as their primary electron acceptor; however, they are phylogenetically distinct from one another (Nevin and Lovely., 2002). G. fermentans are also one of only a few species of freshwater cultivable bacteria that are capable of metal respiration using the electron acceptor Fe (III) oxide. All cells that are grown using Fe (III) oxide or fumarate as an electron acceptor contain c-type cytochromes (Coates et al., 1999).
As an anaerobic chemorganotroph, G. fermentans are well-known for their ability to use iron oxides as well as various other elements as electron acceptors such as Mn (IV), nitrate, fumarate, and the humic acid analog 2, 6 anthraquinone disulfonate, or it can be grown fermentatively with intermediates from the citric or Calvin cycle (Nevin and Lovely, 2002). The Geothrix fermentans H-5 strain for example (the first strain discovered in 1999) is also known for its ability to use several different organic compounds as electron donors such as acetate, propionate, lactate, palmitate, succinate and fumerate; however, it should be noted that Fe (III) should be the electron acceptor in this particular case (Coales et al., 1999). When aquifers become polluted such as in petroleum-contaminated aquifers, Geothrix fermentans become an ideal organism to thrive in these environments because they can use these minerals as electron donors and acceptors to immobilize environmentally harmful radioactive metals such as uranium, technetium, and cobalt. By using minerals such as Fe (III), G. fermentans are able to conserve energy to support growth by coupling the oxidation of organic compounds (Coates et al., 1999). This idea has led to an increase in knowledge for a better understanding of how geochemical cycling of metals within the environment works.
Metal Respiration
Geothrix fermentans is a member of the soil phylum acidobacterium which has been relatively left unstudied over the years. These bacteria are phenotypically and taxonomically diverse from one another and have been found to be located in several different sedimentary ecosystems such as petroleum-contaminated aquifers, fresh-water as well as marine sediments, deep terrestrial sub-surfaces, various soils, and also deep underwater hydrothermal vents that are closely associated with volcanic activity (Coates et al., 1999). Although, G. fermentans itself has been cultivated and studied it has been in large part ignored within the scientific community regarding metal respiration even though it has been recognized in connection with metal-rich, arsenic-releasing, or petroleum-contaminated aquifers as well as other anaerobic communities (Liu et al., 2012). It can be classified as a dissimilatory Fe (III) - reducing bacteria or (DIRB) that conserves its metabolic energy to support growth of the organism via the coupling of the oxidation of organic compounds to the reduction of ferric iron (Coates et al., 1999). H₂ can also be oxidized and coupled to the reduction of ferric acid; however, this process isn’t as common of a process. There is an ongoing debate that the process of dissimilatory Fe (III) reduction could potentially have been an initial attempt by an organism to use oxidative processes for the breakdown of organic compounds because it certainly plays a key role within modern sedimentary environments. G. Fermentans perform several different but equally important functions within sedimentary environments and they include: the oxidation of organic material, the decomposition of iron oxides, and the formation of geologically important compounds such as siderite and magnetite (Coates et al., 1999).
Metal respiration is a type of metabolic process that involves the reduction of insoluble metals such as Fe (III) oxides that act as the dominant electron acceptors releasing protons that will fix carbon into CO₂. Many of the organisms that are associated with this type of metabolic process inhabit sediment environments. This is important because metals and metalloids constitute the majority of the elements that are abundant on earth and these elements can typically be found within sedimentary materials. The reduction of these metals drives the carbon cycles in these types of environments. Even though there are many benefits for using metal respiration, it is a very hard process to master, which is why only a select few species are capable of performing it (Nevin and Lovely., 2002). Microorganisms in the family Geobacteracaea have been shown to be the most prevalent Fe (III)-reducing microorganisms found in sediment settings where Fe (III) reduction is an essential terminal electron acceptor during the process. It has also been shown that most members of the Geobacteracaea family require contact with Fe (III) oxide in order for it to become reduced. When these organisms are not able to come into direct contact with Fe (III) oxide without the assistance of an exogenous electron shuttle they cannot solubilize Fe (III) from Fe (III) oxide before the reduction process (Nevin and Lovely., 2002).
The importance of microorganisms such as Geothrix fermentans over the course of the past two decades have played an important role in understanding certain types of habitats that have been previously misunderstood or not thoroughly explored such as petroleum- contaminated aquifers or hydrothermal vents. The mechanisms by which certain bacteria have evolved to be better suited for certain environments still eludes scientists today; however, by studying these environments and the organisms associated with them we become closer to potentially understanding more rudimentary biological processes than we thought existed. An example involves G. fermentans, unlike the closely associated Geobacteracaea, G. fermentans do not require direct contact with the Fe (III) oxide to perform the reduction process. Although molecular analysis has indicated that the amount of G. fermentans present are much lower compared to the amount of Geobacteracaea, G. fermentans have been found to be much more rich in aquifer sediment environments when it becomes essential for the reduction of Fe (III) to become the ultimate terminal electron-acceptor (Nevin and Lovely., 2002). G. fermentans have been discovered to be the first dissimilatory Fe (III)-reducing microorganism capable of performing Fe (III) reduction by not needing the assistance of an exogenous electron shuttle or by coming into direct contact with insoluble Fe (III) oxide.
Microbial Fuel Cell (MFC)
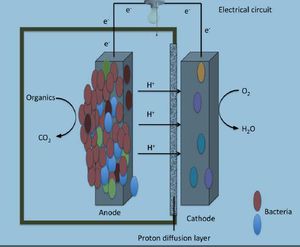
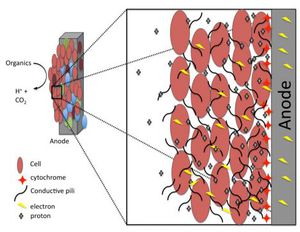

Microbial fuel cells (MFCs) are bio-electrochemical systems that are capable of simulating real life interactions that occur in nature, particularly those involved with decomposing bacteria. MFC's are different from most conventional fuel cells because they do not use chemicals as catalysts for oxidizing methanol or hydrogen (Butler and Nerenberg., 2010). Instead, MFC's use electrochemical microorganisms to catalyze anodic and cathodic reactions. There are two types of MFCs available, those that require a mediator and those that do not require a mediator. A mediator is classified as a chemical that assists the bacterial cell with transporting electrons from its electron transport chain (ETC) to the anode of the MFC. Bacteria that require mediators have been around for a few centuries such as many Proteobacteria, while mediator-less bacteria are a much more recent discovery in the microbial world like Geothrix fermentans.
Reports of harvesting microbial bacteria for their electrical potential have been documented in scientific articles since as early as the 1960’s. Typical designs of electrical generating bacterial experiments include a dense suspension of non-growing bacteria that are mixed with either a redox-active dye, or inorganic compounds such as sulfide. This concoction of non-growing bacteria and dye or inorganic material assists with shuttling small percentages of electrons from the fermentative metabolism process to an electrode surface. An experiment was conducted in 2005 by Bond and Lovely where they showed that there was Evidence for the involvement of an electron shuttle in electricity generation by Geothrix fermentans. In this experiment G. fermentans were cultured with acetate and fumarate. Acetate was used as an electron donor while fumarate was preferentially used as an electron acceptor. The G. fermentans bacterial cells were then collected via centrifugation and resuspended in a similar medium to that which was used initially but excluding both acetate and fumarate. Only 5% of the cells were inoculated into a 250-ml anaerobic glass chamber that used a graphite electrode (62 cm²) as the sole electron acceptor. The medium contained in the chambers was similar to what was used in the growth chambers, however; instead of using fumarate 1mM of acetate was added. The electrodes from both chambers containing one anode and one electrode were connected and flushed with air. The electrodes were connected together via a 500-Ω resistor (Figures 2 and 3). Once the electrode surfaces were fixed with 1% glutaraldehyde they were then dehydrated in buffers with increasing ethanol concentrations. They were then subjected to CO₂ critical-point drying and observed with scanning electron microscopy (SEM) (Figure 4.) G. fermentans cells attached to the electrode surface were observed to have formed a thick coat (Bond and Lovely., 2004) (Figure 4). These findings differed from previous studies involving Proteobacteria because they had shown that individual cells could attach and grow onto the electrode surface without the aid of extracellular material.
The idea of microbial fuel cell (MFC) technology has gained popularity around the world as an ideal alternative renewable power energy supply system. MFCs use the degradation of organic material as a source for fuel (Yusoff et al., 2013). By using bacterial metabolism an electric current can be produced via a variety of organic substrates (Franks and Nevin., 2010). In normal circumstances within the environment when only organic materials are used, Fe (III) would typically be used as the final electron acceptor; however, when attempting to generate electrical power an anode is used as the final electron acceptor by the bacteria. Since there has been shown progress regarding the ability of providing sustainable energy from organic material, interest in this field of research has blossomed over the past decade. Wastewater treatment plants are ideal environments for the use of MFCs as a means of removing biochemical oxygen as well as producing electrical energy. Most research up to this point has focused mainly on wastewater treatment and bioenergy recovery, but there is much optimism in the future for the branching out of MFCs, especially in the area of biosenors (Lefebvre et al., 2011). There recently has been publication of the use of biosenor MFCs for powering oceanography equipment. The Benthic Unattended Generator (BUG) is a biosensor that is ran by the organic matter in aquifer sediments and is one of the first documented biosenors in use.
Conclusion
Geothrix fermentans play a very important role regarding the ecological niches that they encompass. These microorganisms are ideal for their role in purifying petroleum-contaminated aquifers and biogeochemical recycling of metals within the environment. There is still a need for studies to be done on this topic because this species was discovered only 14 years ago. There has been a stagnation in microbial fuel cell development regarding fermentans due to the fact that they use an ETC to shuttle electrons during Fe (III) – reduction (Franks and Nevin., 2010). Due to this ETC shuttle, fermentans do not produce enough power to serve as an ideal renewable energy supply force; however, through future research other valuable uses for G. fermentans could be developed.
References
2. Butler, Caitlyn S., and Robert Nerenberg. "Performance and microbial ecology of air-cathode microbial fuel cells with layered electrode assemblies." Applied microbiology andbiotechnology. 86.5 (2010): 1399-1408. http://link.springer.com/content/pdf/10.1007%2Fs00253-009-2421-x.pdf
5.Lefebvre, Olivier, Arnaud Uzabiaga, In Seop Chang, Byung-Hong Kim, and How Yong Ng. "Microbial fuel cells for energy self-sufficient domestic wastewater treatment—a review and discussion from energetic consideration." Applied microbiology and biotechnology. 89.2 (2011): 259-270. Print. <http://link.springer.com/content/pdf/10.1007/s00253-010-2881-z.pdf>.
6.Mohd Zulkhairi Mohd Yusoff, Anyi Hu, Cuijie Feng, Toshinari Maeda, Yoshihito Shirai, Mohd Ali Hassan, Chang-Ping Yu, Influence of pretreated activated sludge for electricity generation in microbial fuel cell application, Bioresource Technology, Available online 13 March 2013, ISSN 0960-8524, 10.1016/j.biortech.2013.03.003.(http://www.sciencedirect.com/science/article/pii/S0960852413003568
8. [Slonczewski, Joan, and John W. Foster. Microbiology an evolving science. 2nd. ed. New York: W.W Norton & Company, Inc., 2010. 1-1100.]http://biology.kenyon.edu/slonc/Micro/D489Mbio.pdf
9. [Kyrpides, Nikos (September 23, 2011). "Geothrix fermentans DSM 14018". Doe Joint Genome Institute. Retrieved October 26, 2012] http://genomesonline.org/cgi-bin/GOLD/bin/GOLDCards.cgi?goldstamp=Gi11528
10. Liu, Joanne K.; Mehta-Kolte, Misha; Bond, Daniel R. "Expression and purification of GxcA, a c-type cytochrome involved in metal respiration by the bacterium Geothrix fermentans". University of Minnesota. Retrieved October 25, 2012. http://conservancy.umn.edu/bitstream/104494/1/Liu_Joanne.pdf
Edited by Kyle Bailey of Joan Slonczewski for BIOL 238 Microbiology, 2013, Kenyon College.