Leuconostoc mesenteroides
Classification
Higher Order Taxonomic Information
Bacteria; Firmicutes; Bacilli; Lactobacillales; Leuconostocaceae
Species Name
Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293
NCBI: Taxonomy
Species Description
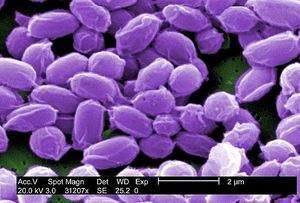
The species is generally viewed as being a cocci, forming long chains or pairs during its growth. However, the morphology can change depending on what media the species is grown on, which can change them to rod shaped or more simply, elongated forms. The cells are Gram positive, which can aid in identification in human pathology. The bacteria is also a nonsporogenous and non-motile species. They are a facultative anaerobe and are a member of the lactic acid bacteria family, which includes Oenococcus oeni, which are also closely related to the Lactobacillaceae (also a LAB), which split off from the Streptococcaceae (Marakova et al. 2006).
Applications and Uses
L. mesenteroides is primarily found upon the skins of a large variety fruits and vegetables. Under the correct micro anaerobic conditions, the L. mesenteroides is actually responsible for beginning the fermentative processes on many standard foods such as kim-chi, sauerkraut, and it even is included in the starter cultures of various breads and dairy cultures (Server-Busson et al. 1999). On sauerkraut specifically, the pH is lowered in the first phase by Klebsiella and Enterobacter species. This lowers the pH so that L. mesenteroides can take hold and further lower the pH. The third and final stage of the fermentation is then taken up by other LABs related to L. mesenteroides (namely, L. plantarum), and lowers the pH even more. (Schieberle 2009). With L. mesenteroides, this process would be missing a niche microbe, and the full fermentation of a commercial product could not be completed without the L. mesenteroides step. This shows the importance that L. mesenteroides has in shaping its own environment as a niche constructor. This feature is also utilized in the meat and dairy industry, as L. mesenteroides produces a protein known as bacterioicin, which prevents the growth of other bacterial species, which is produced by some bacteria as a chemical weapon against a wide range of other species. This is further evidence that L. mesenteroides is a snapper of its environment.
Genome
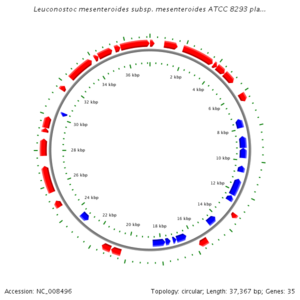
L. mesenteroides has a circular chromosome with 2075763 nucleotides, 2003 protein encoding genes, and 85 RNA genes, around 54% of which have a known purpose. The genome is circular and does contain a variety of plasmids, which aid in citrate metabolism, and bacterioicin development. L. mesenteroides also reproduces by binary fission by first copying its circular genome, and then splitting, and each respective daughter cell gets a copy of the circular genome and plasmids. KEGG Genome
The lactic acid bacteria genomes generally encode for carbon and nitrogen acquisition within their environments. These genes seem to have spawned from the process of horizontal gene transfer as a coevolutionary method from their habitats (Marakova et al. 2006). The L. mesenteroides genome also has relatively few pseudogenes, which number less than 20 on average (Bolotin et al. 2004).
Metabolism
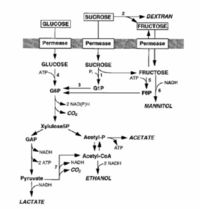
L. mesenteroides is a facultative anaerobe, and under microaerophilic conditions, will undergo a heterolactic fermentation. This results in the break down of glucose and other sugars into D-lactate, ethanol, and CO2 (Demoss et al 1951; Garvie 1986; Gottschalk 1986). It will also convert citrate into diacetyl and acetoin, and will convert sucrose into destrans and levan. Dextrans are used for a variety of different commercial products, some of which include cosmetics, blood plasma extenders, and heparin substitutes (Leathers et al 1995; Sutherland 1996; Alsop 1983; Kim and Day 1994). A variety of polysaccharides are also produced from the metabolic pathways which creates a gel that renders the product unusable, and therefore necessary precautions have to be taken to avoid an accumulation of unwanted metabolite (Tallgren et al. 2006). There are many pathways L. mesenteroides uses in its metabolism which has beginning products of sucrose, fructose, and glucose. Some end products of note are mannitol, acetate, and lactate, but other pathways exist. The schematic to the left shows a brief process of the L. mesenteroides metabolic pathway: G1P, glucose-1-phosphate; G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; GAP, glyceraldehyde-3-phosphate; acetyl-P, acetylphosphate; acetyl-CoA, acetyl coenzyme A; 1, sucrose phosphorylase; 2, dextransucrase; 3,phosphoglucomutase (PGM); 4, glucokinase; 5, fructokinase; 6, mannitol dehydrogenase; 7, pyruvate dehydrogenase.
Pathogenesis and Human Infection
L. mesenteroides is a normal occurrence on fruits and vegetables, and is generally not considered to be an infectious agent in humans. However, there are certain documented instances where L. mesenteroides has actually caused disease within humans. The most surprising of which was a case study where a woman, who was originally thought to have brain tumors, actually had two purulent lesions in her brain that were later successfully identified as an L. mesenteroidies infection (Albanese et al. 2006). Another case is shown where Leuconostoc species are found in cancer patients, and within patients who are critically ill, immunocompromised, or nonsocomial (Cuervo et al. 2008). L. mesenteroides is an opportunistic pathogen, and is not wide-spread in disease, but in more recent years, a large variety of diagnoses have found infections among human patients, meaning it has the potential to infect human hosts. At this time, L. mesenteroides is not generally considered a threat unless in immunosuppressed hosts, and studies are ongoing.
Works Cited
- Albanese A, Spanu T, Sali M, et al. Molecular identification of Leuconostoc mesenteroides as a cause of brain abscess in an immunocompromised patient. J Clin Microbiol. 2006;44:3044–5.
- Alsop, R. M. 1983. Industrial Production of Dextrans. Progress in Industrial Microbiology., 1-42. ed. M. E. Bushell. New York: Elseiver.
- "BacMap: Bacterial Genome Atlas." BacMap: Bacterial Genome Atlas. N.p., n.d. Web. 6 May 2014. <http://wishart.biology.ualberta.ca/BacMap/graphs_cgview.html>
- Bolotin A, Quinquis B, Renault P, Sorokin A, Ehrlich S, Kulakauskas S, Lapidus A, Goltsman E, Mazur M, Pusch G, et al. “Less than 20 pseudogenes.” Nat. Biotechnol. 2004;22:1554–1558.
- Demoss, R. D., R. C. Bard, and I. C. Gunsalus. 1951. The mechanism of heterolactic fermentation: a new route of ethanol formation. J. Bacteriol. 62: 499-511.
- M Dols, W Chraibi, M Remaud-Simeon, N D Lindley, and P F Monsan. 1997. Growth and energetics of Leuconostoc mesenteroides NRRL B-1299 during metabolism of various sugars and their consequences for dextransucrase production. Appl. Environ. Microbiol. June 1997 63:6 2159-65.
- Garvie, E. I. 1986. Genus Leuconostoc. Bergey's Manual of Systematic Bacteriology. eds. P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt. Baltimore, MD: The Williams and Wilkins Co.
- Gottschalk, G. 1986. Bacterial Metabolism. 2nd ed. New York: Springer-Verlag.
- Kim, D., and D. F. Day. 1994. A new process for the production of clinical dextran by mixed-culture fermentation of Lipomyces starkeyi and Leuconostoc mesenteroides. Enzyme Microb. Technol. 16: 844-48.
- Leathers, T. D., G. T. Hayman, and G. L. Cote. 1995. Rapid Screening of Leuconostoc mesenteroides Mutants for Elevated Proportions of Alternan to Dextran. Current Microbiol. 31: 19-22.
- Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, et al. (2006) Comparative genomics of the lactic acid bacteria. Proceedings of the National Academy of Sciences of the United States of America 103: 15611–15616.
- Schieberle, Peter (2009). The pH of completely cured sauerkraut is about 3.6; see Belitz, H.-D.; Grosch, Werner; Food Chemistry (4th Edition). Springer. p. 803.
- Server-Busson, C., C. Foucaud, and J.-Y. Leveau. 1999. Selection of Dairy Leuconostoc Isolates for Important Technological Properties. J. Dairy Res. 66: 245-56.
- Sutherland, I. W. 1996. Extracellular Polysaccharides. 2nd ed. Biotechnology, eds. H.-J. Rehm, G. Reed, A. Puhler, and P. Stadler, Vol 6: Products of Primary Metabolism. New York: VCH.
- Tallgren, A. H., U. Airaksinen, R. von Weissenberg, H. Ojamo, J. Kuusisto, and M. Leisola. 1999. Exopolysaccharide-Producing Bacteria from Sugar Beets. Appl. Environ. Microbiol. 65, no. 2: 862-64.
Author
Page authored by Chandler F. Divan, student of Prof. Jay Lennon at Indiana University- Bloomington.