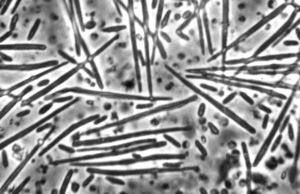
Metschnikowia bicuspidata
Description and Significance
Metschnikowia are single-celled fungal parasites of freshwater animals [7]. It typically parasitises crustaceans, including Daphnia, a genus of zooplankton [7]. M. bicuspidata has also been found to infect prawns and salmon [6].
The organism has two morphological forms: round vegetative cells and single-celled needle-shaped propagative spores [7]. M. bicuspidata uses energy from the host Daphnia to produce tens of thousands of identical haploid spores. These increase in number until the Daphnia is killed and its carapace ruptures, introducing tens of thousands of new spores into the water column.
Daphnia and M. bicuspidata are used in ecology and evolutionary biology to study ecological phenomena in aquatic ecosystems. M. bicuspidata is used to study host-parasite dynamics and the effects of parasitism on host community evolution.
Classification
Eukaryota, Fungi, Dikarya, Ascomycota, Saccharomycotina, Saccharomycetes, Saccharomycetales, Metschnikowiaceae, Metschnikowia (European Nucleotide Archive, accessed 21 April 2015)
There are three varieties in the species: M. bicuspidata var. bicuspidata, var. californica, and var. chathamia [7].
Genome Structure
The genome of Metschnikowia bicuspidata NRRL YB-4993 is 16.06 Mbp long. Being a eukaryote, this parasite's DNA is linear. This genome of this species contains 1376 genes associated with cellular processes and signaling, 1088 genes associated with information storage and processing, 1237 genes associated with metabolism, and 877 genes that are poorly characterized.
26S statistics for M. bicuspidata var. bicuspidata show an average similarity between strains to be 99.3%, while var. chathamia has an average similarity of 98.6% between strains.
Cell Structure, Metabolism and Life Cycle
M. bicuspidata is a chemoorganoheterotroph, and uses energy from the Daphnia body to reproduce [5].
M. bicuspidata variants can be determined by their metabolic activities. M. bicuspidata var. californica has the ability to assimilate methyl-α-D-glucoside, D-gluconate and s-Keto-D-gluconate. M. bicuspidata var. chathamia can assimiliate methyl-α-D-glucoside, but cannot assimilate D-gluconate or 2-keto-D-gluconate. M. bicuspidata var. bicuspidata is unable to assimilate D-glucoside.
The life cycle proceeds as follows: Needle-shaped cells (haploid, n) float in the water column and are eaten by Daphnia. In the Daphnia hemolymph, these cells reproduce asexually to form spherical vegetative cells (haploid, n) that undergo plasmogamy (forming one cell containing two separate haploid nuclei. written in shorthand: n + n). After a delay, karyogamy occurs, resulting in a single diploid cell (2n). This diploid cell may undergo asexual mitotic haploidization, yielding vegetative haploid spherical cells. Alternatively, the diploid cell may undergo sexual meiotic haploidization, yielding haploid propagative needle-shaped ascospores [7]. The ascospores are the form that persist in the water column and infect hosts [7]. The sharp, needle-like shape of the propagative spores suggest a puncture mechanism for entering through the Daphnia gut wall.
Ecology and Pathogenesis
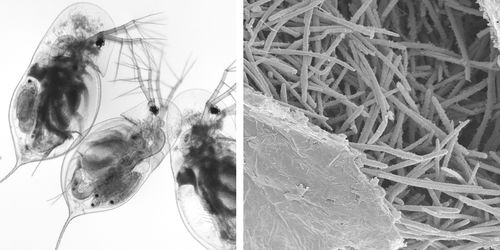
Exposure of host Daphnia to parasitic M. bicuspidata occurs during feeding. Daphnia eat algae in the water column and feed indiscriminately on whatever floating particles fit into their mouths. During feeding, M. bicuspidata spores enter the Daphnia mouth, travel along the gut, and puncture the gut wall. Infection occurs when a spore punctures the wall, enters the hemolymph, and begin reproducing [4].
M. bicuspidata kills the host D. magna in 7-25 days (average = 17.5 days); a healthy D. magna typically lives 40-80 days [4].
Virulence depends on the quality of food eaten by the Daphnia [5]. Daphnia die more quickly from infection when they are fed more, better quality food than when they are fed very little or low-quality food [5].
Susceptibility to infection varies across host genotypes. Infected D. magna exhibit reduced fecundity [4]. Upon death due to infection, Daphnia may yield 10,000 to 70,000 spores per individual [8].
Transmission is horizontal, meaning infection occurs within a host generation and is not transmitted from parent to offspring [4].
M. bicuspidata can survive at temperatures of 9-27 degrees C, salinity of 270 ppt, and NaCl concentrations of 0-180 ppt [6]. (For comparison, sea water salinity is approximately 35 ppt).
Infection in other animals, for example, prawn and salmon, produces symptoms including altered tissue coloration, edema, inflammation in muscles, swollen organs, necrotic lesions, and early death [2][3]. The prawn contained yeast at approximately 10(8) to 10(9) colony forming units per 100 mg of tissue [3].
References
[6] Moore, M., and Strom, M. "Infection and mortality by the yeast Metschnikowia bicuspidata var. bicuspidata in chinook salmon fed live adult brine shrimp (Artemia franciscana)." Aquaculture. 2003. Volume 220(1-4). pp. 43-57. DOI: 10.1016/S0044-8486(02)00271-5.
Author
Page authored by Katie Griebel and Jacob Gelarden, students of Prof. Jay Lennon at Indiana University.