Chest Port Microbial Infections
Section
By [Hannah Lorico Hertz]
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
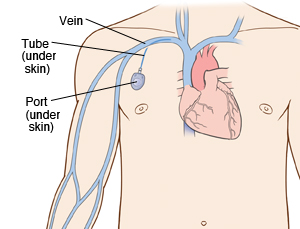
A chest port is a catheter connected to a reservoir inserted under the skin of the chest and used to administer medicines directly into a vein over a long period of time. Chest ports are commonly used to administer long-term chemotherapy in children because of the ease of care for port maintenance. In comparison to an IV line, chest ports can stay in place for months at a time, can be used to collect blood samples without needles, and have a lower risk of infection over time.
Although port infections are not as common as other catheter infections, microbial infections are still the most significant complication resulting in port excision. About 5% of patients require port excision because of infection.[1] Infections of implanted devices most commonly result from Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Streptococcus vidrians, Klebsiella pneumonia, and Pseudomona aeruginosa. [2] Of the above microbes, S. epidermidis is the most relevant port associated pathogen. In the United States, Jukes et al. estimate 80% of nosocomial catheter related bloodstream infections (CRBSI) are a result of S. epidermidis. [3]
S. epidermidis is a natural member of the human skin flora[4]. Under normal conditions, S. epidermidis is not pathogenic. S. epidermidis only acts as a human pathogen in individuals with compromised immune systems, immunosuppression, or chemotherapy related neutropenia[5].
Common port infections include S. epidermidis biofilm formation inside the catheter lumen.[6] Biofilm formation is threefold. S. epidermidis adhere to the catheter surface to be colonized, a microcolony forms, and S. epidermidis cells detach from a mature biofilm allowing S. epidermidis colonization on additional body sites.
Better management of chest port microbial infections can help improve patient quality of life as they undergo treatment. Recent research explores how to best manage port microbial infections avoiding the current treatment of port excision.
Sample citations: [7]
[8]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Section 1
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
Section 2
Include some current research, with at least one figure showing data.
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ Funaki, Brian. “Subcutaneous Chest Port Infection.” Seminars in Interventional Radiology, 22.3 (2005): 245–247. PMC.
- ↑ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" Biomed Microdevices, 16(2014): 365.
- ↑ Jukes, L., Mikhail, J., Bome-Mannathoko, N., Hadfield, S.J., Harris, L.G., El-Bouri, K., Davies, A.P., Mack, D. "Rapid differentiation of Staphylococcus aureus, Staphylococcus epidermidis and other coagulase-negative staphylococci and meticillin susceptibility testing directly from growth-positive blood cultures by multiplex real-time PCR" J. Med. Microbiol, 59 (2010):1456–1461
- ↑ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of Staphylococcus Epidermidis Biofilm Formation: Mechanisms and Molecular Interactions." Frontiers in Cellular and Infection Microbiology 5 (2015): 14. PMC.
- ↑ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of Staphylococcus Epidermidis Biofilm Formation: Mechanisms and Molecular Interactions." Frontiers in Cellular and Infection Microbiology 5 (2015): 14. PMC.
- ↑ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" Biomed Microdevices, 16(2014): 365.
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
