Chest Port Microbial Infections
Introduction
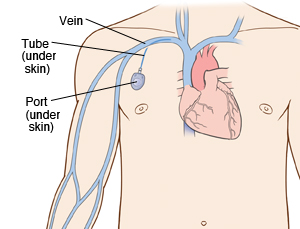
By Hannah Lorico Hertz
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
A chest port is a catheter connected to a reservoir inserted under the skin of the chest and used to administer medicines directly into a vein over a long period of time. Chest ports are commonly used to administer long-term chemotherapy in children because of the ease of care for port maintenance. In comparison to an IV line, chest ports can stay in place for months at a time, can be used to collect blood samples without needles, and have a lower risk of infection over time.
Although port infections are not as common as other catheter infections, microbial infections are still the most significant complication resulting in port excision. About 5% of patients require port excision because of infection.[1] Infections of implanted devices most commonly result from Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Streptococcus vidrians, Klebsiella pneumonia, and Pseudomona aeruginosa. [2] Of the above microbes, S. epidermidis is the most relevant port associated pathogen. In the United States, Jukes et al. estimate 80% of nosocomial catheter related bloodstream infections (CRBSI) are a result of S. epidermidis. [3]
S. epidermidis is a natural member of the human skin flora[4]. Under normal conditions, S. epidermidis is not pathogenic. S. epidermidis only acts as a human pathogen in individuals with compromised immune systems, immunosuppression, or chemotherapy related neutropenia[5].
Common port infections include S. epidermidis biofilm formation inside the catheter lumen.[6] Biofilm formation is threefold. S. epidermidis adhere to the catheter surface to be colonized, a microcolony forms, and S. epidermidis cells detach from a mature biofilm allowing S. epidermidis colonization on additional body sites.
Better management of chest port microbial infections can help improve patient quality of life as they undergo treatment. Recent research explores how to best manage port microbial infections avoiding the current treatment of port excision.
Sample citations: [7]
[8]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Biofilm Formation
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
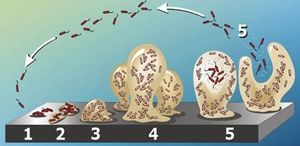
Staphylococcus epidermidis biofilm formation begins with cell attachment to a surface (Figure 2). For port microbial infections, biofilm formation most commonly occurs on the catheter lumen. Next, S. epidermidis begin producing polysaccharides forming a protective layer surrounding the cells. The biofilm continues to grow until the cells are released into the environment, free to begin biofilm formation at another surface. [9]
The primary attachment of S. epidermidis on the surface of the port catheter lumen is essential for the establishment of port-associated infections. Heilmann et al. (1997) [10] identified factors essential for S. epidermidis-surface interactions. The autolysin AtlE is essential for S. epidermidis attachment to polystyrene (plastic).[11] Moreover, as foreign materials are inserted into the body, they become covered by host extracellular matrix components (ECM) and so, as chest ports are inserted in the body, the ports become covered by EMC such as vitronectin and collagen. Research has shown that proteins with ECM-binding activity expressed by S. epidermidis can play a significant role in microbial biofilm initiation of a port infection. Proteins such as AltE bind to vitronectin, lipase GehD to collagen [12] , and surface component serine-asparatate repeat (Sdr) [13] proteins SdrF, SdrG, and SdrH to [14] to collagen I [15], fibrogen, and fibrinogen respectively.
Biofilm accumulation of S. epidermidis begins with the expression of intercellular adhesive properties. The protein, polysaccharide intercellular adhesin (PIA), is the main adhesive expressed by S. epidermidis [16] leading to cell aggregation and biofilm formation. [17] Proteins Aap and Embp also lead to biofilm assembly. Other biofilm associated proteins involved with biofilm accumulation include SesC. [18]
The understanding of S. epidermidis pathogenicity is linked to biofilm formation. Thus, many studies have been conducted studying biofilm formation in vitro. Further research of biofilm systems in complex models are necessary to more accurately portray the in vivo infection of a chest port.
Symptoms and Diagnosis of Infection
Include some current research, with at least one figure showing data.
Chest port infections most commonly occur in patients with compromised immune systems, immunosuppression, and chemotherapy related neutropenia. The onset of a port infection can be recognized by numerous symptoms. Symptoms include a high fever (≥ 38.3°C or 101°F), redness, tenderness, and induration at port site. With neutropenic patients, sometimes the only detectable symptom of a port infection is fever and chills because of a lack of inflammatory responses.( Sanjeet Dadwal,2015)
The diagnostic workup of an infection includes at least two blood cultures, one from a peripheral vein and one from the port catheter, stool examination for C. difficile and other bacterial/protozoal microbes (if diarrhea is present),urine culture and urinalysis, a chest radiograph, respiratory samples for culture, and the aspiration or biopsy of any skin lesions.
In a case study by Feroz Pasha, Amita Mahajan, Sameer Kaul, and Rohit V. Nayyar, a pediatric oncology patient with a pocket infection was treated with intravenous broad-spectrum antibiotics. However, two patients in their study developed line sepsis. To treat this infection, the doctors removed these patients' ports.
Current Treatments
Include some current research, with at least one figure showing data.
Section 4
Conclusion
Bold
Italic
Subscript: H2O
Superscript: Fe3+
References
- ↑ Funaki, Brian. “Subcutaneous Chest Port Infection.” Seminars in Interventional Radiology, 22.3 (2005): 245–247. PMC.
- ↑ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" Biomed Microdevices, 16(2014): 365.
- ↑ Jukes, L., Mikhail, J., Bome-Mannathoko, N., Hadfield, S.J., Harris, L.G., El-Bouri, K., Davies, A.P., Mack, D. "Rapid differentiation of Staphylococcus aureus, Staphylococcus epidermidis and other coagulase-negative staphylococci and meticillin susceptibility testing directly from growth-positive blood cultures by multiplex real-time PCR" J. Med. Microbiol, 59 (2010):1456–1461
- ↑ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of Staphylococcus Epidermidis Biofilm Formation: Mechanisms and Molecular Interactions." Frontiers in Cellular and Infection Microbiology 5 (2015): 14. PMC.
- ↑ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of Staphylococcus Epidermidis Biofilm Formation: Mechanisms and Molecular Interactions." Frontiers in Cellular and Infection Microbiology 5 (2015): 14. PMC.
- ↑ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" Biomed Microdevices, 16(2014): 365.
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
- ↑ 2007
- ↑ Heilmann C., Hussain M., Peters G., Götz F. "Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface." Mol. Microbiol. (1997) 24, 1013–1024.
- ↑ Bowden M. G., Visai L., Longshaw C. M., Holland K. T., Speziale P., Hook M. "Is the GehD lipase from Staphylococcus epidermidis a collagen binding adhesin?" (2002). J. Biol. Chem. 277: 43017–43023.
- ↑ Bowden M. G., Visai L., Longshaw C. M., Holland K. T., Speziale P., Hook M. "Is the GehD lipase from Staphylococcus epidermidis a collagen binding adhesin?" (2002). J. Biol. Chem. 277: 43017–43023.
- ↑ McCrea K. W., Hartford O., Davis S., Eidhin D. N., Lina G., Speziale P., et al. "The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis." . (2000) Microbiology 146: 1535–1546.
- ↑ E., McCrea K. W., Ni E. D., O'Connell D., Cox J., Hook M., Foster, T.J. "Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus." (1998). Microbiology 144:3387–3395.
- ↑ Arrecubieta C., Asai T., Bayern M., Loughman A., Fitzgerald J. R., Shelton C. E., Baron, H.M., Dang, N.C., Deng, M.C., Naka,Y., Foster, T.J., Lowy, F.D. "The role of Staphylococcus aureus adhesins in the pathogenesis of ventricular assist device-related infections." (2006) J. Infect. Dis. 193:1109–1119.
- ↑ [Mack D., Siemssen N., Laufs R. "Identification of a cell cluster associated antigen specific for plastic-adherent Staphylococcus epidermidis which is functional related to intercellular adhesion. (1994b) Zentralbl. Bakteriol. Suppl. 26: 411–413.]
- ↑ Costerton J. W., Lewandowski Z., Caldwell D. E., Korber D. R., Lappin-Scott H. M. "Microbial biofilms." (1995). Annu. Rev. Microbiol. 49: 711–745.
- ↑ Shahrooei M., Hira V., Stijlemans B., Merckx R., Hermans P. W., Van Eldere J. " Inhibition of Staphylococcus epidermidis biofilm formation by rabbit polyclonal antibodies against the SesC protein." (2009) Infect. Immun. 77: 3670–3678.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
