The Gut Microbiome and Parkinson's Disease
by Rebecca Hölzel
An introduction to Parkinson's Disease can be found here
Abnormal Gut Microbiome
The conditions that contribute to the development of Parkinson's Disease are likely very complex. A growing body of research has indicated, however, that the gut microbiome may play a key role in disease development and progression. Parkinson's Disease is commonly associated with abnormal gut microbiomes, including increased and decreased counts of bacteria normally found in healthy individuals.[2] However, some researchers remain skeptical that the gut microbiome influences Parkinson's Disease, believing instead that the disease is what causes the abnormal gut bacteria, and not the abnormal gut bacteria that cause the disease.[3]
General Abnormalities
One research team examined the relationship between gut microbiota and Parkinson's Disease by recruiting newly diagnosed and unmedicated Parkinson's disease patients for study. Using antibiotics or having other gut-related diseases constituted grounds for expulsion. Fecal samples were collected from all patients and used to extract bacterial DNA, which provided measures of bacterial diversity and relative abundance of bacterial genera. They discovered that levels of Verrucomicrobia, Verrucomicrobiaceae, and Akkermansia were twofold higher in Parkinson's Disease patients than healthy controls. Levels of Proteobacteria, Enterobacteriaceae, Christensenellaceae, Lactobacillaceae, Coriobacteriaceae, Bifidobacteriaceae, and Parabacteroides also increased in Parkinson's Disease patients, with Roseburia showing a considerable decrease. A significant increase was also seen for Oscillospira and a significant decrease was seen for Ruminococcus.[4]
Gut Inflammation
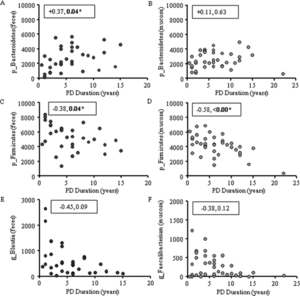
Intestinal inflammation, common to Parkinson's Disease, has been linked to abnormal gut microbiota through characterization of bacteria as either pro-inflammatory or anti-inflammatory. It has been found that healthy individuals possess far more colonic mucosal bacteria from Coprobacillaceae, Dorea, and Faecalibacterium than Parkinson's Disease patients. Notably, Faecalibacterium have anti-inflammatory properties. Parkinson's Disease patients also have more pro-inflammatory bacteria, including bacteria from the family Oxalobacteraceae. Fecal samples have also shown higher levels of pro-inflammatory bacteria, including Akkermansia, Oscillospira, and Bacteroides. Levels of pro-inflammatory and anti-inflammatory bacteria correlate to Parkinson's Disease duration.[1]
Phenotypes
One research team attempted to connect abnormal gut microbiota with common Parkinson's Disease phenotypes (tremor, abnormal gait, poor balance). From fecal samples, they discovered that the mean abundance of Prevotellaceae was 77.6% lower in Parkinson's Disease patients than in healthy controls. By applying generalized linear modeling, the team demonstrated that this difference could not be explained by differences in constipation levels, comorbidities, or medications. Interestingly, their models revealed that increased levels of Ruminococcaceae in Parkinson's Disease patients could be explained by the decrease in Prevotellaceae; that is, Ruminococcaceae increased because Prevotellaceae decreased. When the researchers attempted to link gut microbiota with phenotype, they discovered that Enterobacteriaceae were more abundant in patients who dominantly displayed abnormal gait/poor balance than patients who dominantly displayed tremors. They speculated that increased levels of Enterobacteriaceae may explain why patients who do not express the tremor phenotype have faster disease progression and worse prognosis.[5]
Another team discovered that Parkinson's Disease patients with higher abundance of Christensenellaceae had worse nonmotor symptoms. Increased Lactobacillaceae and decreased Lachnospiraceae also correlated with worse cases of intellectual impairment, nonmotor symptoms, gait instability, and posture problems.[4]
Bacterial Overgrowth
Parkinson's Disease has been associated with general overgrowth of bacteria in the small intestine (SIBO, small intestinal bacterial overgrowth). One study tested for SIBO in 48 Parkinson's Disease patients compared to 36 healthy controls. All participants were tested using a hydrogen glucose breath test. The researchers found a significant number of Parkinson's Disease patients (26/48 participants) had SIBO compared to the controls (3/36 participants). Furthermore, Parkinson's Disease patients with SIBO were more likely to report GI symptoms such as bloating and flatulence. Disease duration and severity also correlated with a diagnosis of SIBO.[6]
Drug Interactions
L-Dopa
Levodopa (L-dopa) is widely used in the treatment of Parkinson's Disease. Despite its efficacy, up to 56% of administered L-dopa fails to reach the brain because it is metabolized in other pathways. Gut bacteria are suspected to play a large role in loss of L-dopa function; as such, identifying any microbes responsible for metabolizing L-dopa is crucial.[7]
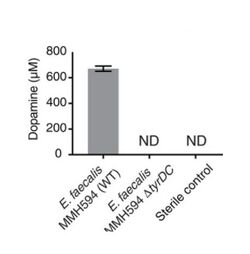
Genome mining was used to identify a protein, tyrosine decarboxylase (TyrDC), in human gut Enterococcus faecalis capable of decarboxylating L-dopa and producing dopamine. TyrDC's role was confirmed by experiments that found knockout of the TyrDC gene drastically reduced Enterococcus faecalis dopamine production. Furthermore, enzyme assays with TyrDC using both L-dopa and the preferred substrate tyrosine showed that TyrDC was capable of decarboxylating both substrates simultaneously, indicating that substrate competition would not hinder TyrDC's affinity for L-dopa.[7]
It has also been proposed that Helicobacter pylori may decrease L-dopa bioavailability. Initial experiments have shown that laboratory Helicobacter pylori can grow quickly on L-dopa, suggesting that they may consume L-dopa when present in the gut.[8]
Treatment Options
The potential link between Parkinson's Disease and gut microbiota opens the possibility of related treatments.
Fecal Transplants
A 71 year old male Parkinson's Disease patient experienced symptom relief after a fecal transplant. Prior to the transplant, the patient suffered extreme constipation not relieved by laxatives, in addition to symptoms of tremor and bradykinesia (slow movements). Due to the severity of symptoms, the patient agreed to undergo three days of fecal transplantation using stool from a healthy 26-year-old male. A week after the treatment, the patient reported complete disappearance of leg tremors. Although the tremors returned after two months, the severity was greatly decreased. The constipation was also greatly relieved, although the patient reported no change in face or neck stiffness.[9]
Researchers sampled the patient's stool to characterize his gut microbiota both before and after the fecal transplants. After one week, the patient's gut microbiota were similar to the stool donor's gut microbiota, with increases in Firmicutes and decreases in Proteobacteria and Bacteroidetes. There were also increases in Lachnoclostridium, Dialister, Alistipes, and Unidentified-Ruminococcaceae. After one month, there was an increase in Megamonas and after 3 months, Akkermansia and Faecalibacterium increased.[9]
Another team recruited 11 Parkinson's Disease patients to further study the possibility of fecal transplant as a treatment option. Symptoms, as well as gut microbiota composition, were assessed prior to treatment, 6 weeks after treatment, and 12 weeks after treatment. One of the parameters of the study included rating patients on the Wexner constipation scale. After 6 weeks, patients reported significantly lower constipation scores, although these scores had increased again by the 12-week mark. Patients also reported improvements in posture and gait.[10]
After fecal transplant, the abundance of Blautia and Lachnospiraceae increased for the Parkinson's Disease patients. Compared to healthy controls, there was an increased abundance of Bacteroides and decreased abundance of Faecalibacterium in Parkinson's Disease patients, both before and after the fecal transplant. There was also an increased abundance of Enterobacteriaceae in Parkinson's Disease patients compared to the healthy controls, but this abundance decreased after fecal transplant.[10]
Probiotics
In one study, Parkinson's Disease patients who consumed fermented milk with probiotics and prebiotics reported greater relief from constipation symptoms compared to a placebo group.[12]
In another study, researchers evaluated the effectiveness of probiotics in relieving Parkinson's Disease-related GI symptoms compared to trimebutine (a drug commonly used in GI disorders, such as irritable bowel syndrome, to relax the muscles of the intestines). Patients that received trimebutine reported improvements in abdominal pain, bloating, and constipation. Patients that received probiotics reported statistically significant improvements in only abdominal pain and bloating. However, researchers found it promising that probiotics could treat Parkinson's Disease symptoms nearly as well as trimebutine.[13]
Parkinson's Disease patients that took probiotics containing Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum for 12 weeks had decreased scores on the Movement Disorders Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS). They also had decreased high-sensitivity C-reactive protein (hs-CRP) values, which indicate acute inflammation in the body. Other notable results from this study included decreased insulin levels and decreased triglyceride levels.[14]
Antibiotics
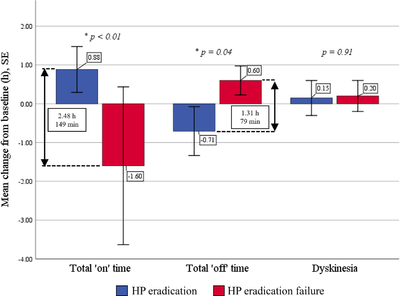
Antibiotics may improve the symptoms of Parkinson's Disease. One study enrolled 22 Parkinson's Disease patients also suffering from Helicobacter pylori infection. After two weeks of treatment with amoxicillin, clarithromycin, and omeprazole, 17 of the patients experienced complete eradication of Helicobacter pylori. They reported statistically significant increases in total "on" time per day and decreases in "off" time per day, as well improvements in tremor, mood, and GI symptoms. Patients who did not experience complete eradication reported no improvements and an increase in "off" time per day.[11]
References
- ↑ 1.0 1.1 Keshavarzian,Ali, Stefan J. Green, Phillip A. Engen, Robin M. Voigt, Ankur Naqib, Christopher B. Forsyth, Ece Mutlu, Kathleen M. Shannon. (2015). "Colonic bacterial composition in Parkinson's disease." Movement Disorders, vol. 30(10), 1351-1360. https://doi.org/10.1002/mds.26307
- ↑ Sampson, Timothy. (2020). "The Impact of Indigenous Microbes on Parkinson's Disease." Neurobiology of Disease, vol. 135. https://doi.org/10.1016/j.nbd.2019.03.014
- ↑ Quigley, Eamonn. (2017). "Gut microbiome as a clinical tool in gastrointestinal disease management: are we there yet?" Nature Reviews Gastroenterology & Hepatology, vol. 14, 315-320. https://doi.org/10.1038/nrgastro.2017.29
- ↑ 4.0 4.1 Barichella, M., Severgnini, M., Cilia, R., Cassani, E., Bolliri, C., Caronni, S., Ferri, V., Cancello, R., Ceccarani, C., Faierman, S., Pinelli, G., De Bellis, G., Zecca, L., Cereda, E., Consolandi, C. and Pezzoli, G. (2019). "Unraveling Gut Microbiota in Parkinson's Disease and Atypical Parkinsonism. Movement Disorders, vol. 34, 396-405. https://doi.org/10.1002/mds.27581
- ↑ Scheperjans, Filip, Velma Aho, Pedro A. B. Pereira, Kaisa Koskinen, Lars Paulin, Eero Pekkonen, Elena Haapaniemi, Seppo Kaakkola, Johanna Eerola-Rautio, Marjatta Pohja, Esko Kinnunen, Kari Murros, Petri Auvinen. (2014). "Gut microbiota are related to Parkinson's disease and clinical phenotype." Movement Disorders, vol. 30(3), 350-358. https://doi.org/10.1002/mds.26069
- ↑ Gabrielli, Maurizio, Patrizia Bonazzi, Emidio Scarpellini, Emanuele Bendia, Ernesto C. Lauritano, Alfonso Fasano, Maria G. Ceravolo, Marianna Capecci, Anna Rita Bentivoglio, Leandro Provinciali, Pietro A. Tonali, Antonio Gasbarrini. (2011). "Prevalence of Small Intestinal Bacterial Overgrowth in Parkinson's Disease." Movement Disorders, vol. 26(5), 889-892. https://doi.org/10.1002/mds.2356
- ↑ 7.0 7.1 7.2 Rekdal, Vayu, Elizabeth Bess, Jordan Bisanz, Peter Turnbaugh, & Emily Balskus. (2019). "Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism." Science, vol. 364(6445). 10.1126/science.aau6323
- ↑ Lyte, Mark. (2010). "Microbial endocrinology as a basis for improved l-DOPA bioavailability in Parkinson’s patients treated for Helicobacter pylori." Medical Hypotheses, vol. 74(5), 895-897. https://doi.org/10.1016/j.mehy.2009.11.001
- ↑ 9.0 9.1 Huang, Hongli, Haoming Xu, Qingling Luo, Jie He, Mengyan Li, Huiting Chen, Wenjuan Tang, Yuqiang Nie, & Yongjian Zhou. (2019). "Fecal microbiota transplantation to treat Parkinson's disease with constipation." Medicine (Baltimore), vol. 98(26). 10.1097/MD.0000000000016163
- ↑ 10.0 10.1 Kuai, Xiao-yi, Xiao-han Yao, Li-juan Xu, Yu-qing Zhou, Li-ping Zhang, Yi Liu, Shao-fang Pei & Chun-li Zhou. (2021). "Evaluation of fecal microbiota transplantation in Parkinson's disease patients with constipation." Microbial Cell Factories, vol. 20(98). https://doi.org/10.1186/s12934-021-01589-0
- ↑ 11.0 11.1 Lolekha, Praween, Thanakarn Sriphanom, & Ratha-Korn Vilaichone. (2021). Helicobacter pylori eradication improves motor fluctuations in advanced Parkinson’s disease patients: A prospective cohort study (HP-PD trial)." PLoS ONE. https://doi.org/10.1371/journal.pone.0251042
- ↑ Barichella, Michela, Claudio Pacchetti, Carlotta Bolliri, Erica Cassani, Laura Iorio, Chiara Pusani, Giovanna Pinelli, Giulia Privitera, Ilaria Cesari, Samanta Andrea Faierman, Riccardo Caccialanza, Gianni Pezzoli, & Emanuele Cereda. (2016). "Probiotics and prebiotic fiber for constipation associated with Parkinson disease." Neurology, vol. 87(12). https://doi.org/10.1212/WNL.0000000000003127
- ↑ Georgescu, Doina, Oana Elena Ancusa, Liviu Andrei Georgescu, Ioana Ionita, & Daniela Reisz. (2016). "Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: is there hope?" Clinical Interventions in Aging, vol. 11, 1601–1608. 10.2147/CIA.S106284
- ↑ Omid Reza Tamtaji, Mohsen Taghizadeh, Reza Daneshvar Kakhaki, Ebrahim Kouchakia, Fereshteh Bahmani, Shokoofeh Borzabadi, Shahrbanoo Oryan, Alireza Mafi, & Zatollah Asemi. (2019). "Clinical and metabolic response to probiotic administration in people with Parkinson's disease: A randomized, double-blind, placebo-controlled trial." Clinical Nutrition, vol. 38(3), 1031-1035. https://doi.org/10.1016/j.clnu.2018.05.018
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
