Probiotic Lactobacillus and Promotion of Tumor Growth
Logan Gusmano
Microbio Wiki
Introduction
Lactobacillus, a Gram-positive, non-spore forming firmicute, rod-shaped Bacilli bacteria, inhabiting mammalian gastrointestinal tract microbiota. Lactobacillus, sometimes referred to as Acidophilus, is a common probiotic that promotes the digestion and breakdown of lactose in a healthy gut microbiome. https://www.mayoclinic.org/drugs-supplements-acidophilus/art-20361967
Lactobacillus is further speciated by its metabolic pathways: obligately facultatively homofermentative, and obligately heterofermentative. Both pathways are classified by unique types of metabolic products. Obligately facultatively homofermentative Lactobacilli ferment a majority of hexoses into lactic acid, oxidizing the molecules for chemical energy. Whereas, heterofermentative Lactobacilli glucose fermentation not only produces lactic acid, but also ethanol and carbon dioxide, oxidizing the molecules for chemical energy.
Classified as a lactic acid bacterium (LAB, Leuconostocaceae) https://www.mayoclinic.org/drugs-supplements-acidophilus/art-20361967, resistant in low pH environments, Lactobacilli tolerate high proton concentration in the mammalian gut microbiome, proliferating and breaking down lactose into metabolite lactic acid. Lactobacillus is considered a common microbe consumed by mammals as fermented products, cheese, wine, and dairy. Identifying the impact of lactic acid to antitumor defenses, novel research identified Lactobacillus’ capability of promoting detrimental effects to the host's immune defenses targeting tumor proliferation. In a recently published paper from Vrije Universiteit Brussel, one of the byproducts of Lactobacillus’ metabolism, lactic acid, has the molecular capacity to weaken the antitumor defenses of mammals, initiating macrophages as a tumor promoter in the body. https://www.sciencedaily.com/releases/2022/01/220128141333.htm
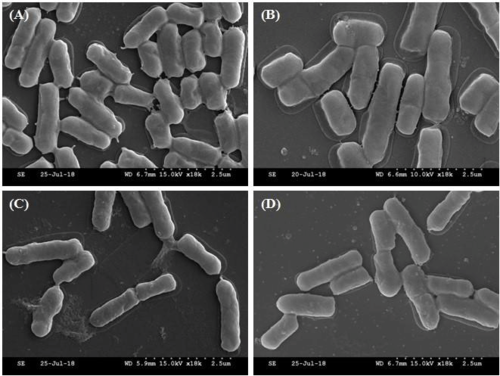
Scanning Electron Microscopy image (SEM), of selected Lactobacilli strain MG4221, MG4231, MG4261,
and MG4270. (1-20 nm) https://www.researchgate.net/figure/The-scanning-electron-microscope-SEMimages-of-selected-strainsA-Lactobacillus_fig1_332622236
Classification and Biological Structure
Cell Wall
Gram-positive bacteria Lactobacillus comprise of peptide bonded glycopolymers and integral membrane bound proteins complex structures. https://pubmed.ncbi.nlm.nih.gov/25186919/ This consists of a multi-polymer chain of peptidoglycan sacculus that covers the inner cell membrane, and a cytoplasmic membrane regulating the osmotic pressure around the cell. These components of the membrane are significant to better understand the microbes capability of cell division and proliferation, ability to interact with its external environment, and ability of defending itself from external factors. However, despite sharing biological structure similarities with Gram-positive bacteria, Lactobacillus cell wall demonstrates unique properties of extremophiles differentiating it from other Gram-positive bacilli. For instance, Lactobacillus’ cell wall contains several membrane receptors significant in the binding of bacteriophages, preventing significant fermentation processes in the food industry. The presence of Type IV pili present on Lactobacillus that act as adhesive factors, allowing it to proliferate in the gut microbiota of organisms. Secondarily, Lactobacillus’ adhesive properties are derived from S-layer protein allowing it to bind to the dendritic specific site of human dendritic cells, modulating the immune response of the host. Cell wall structure and function in lactic acid bacteria - PubMed (nih.gov) The S-layer of the microbial surface of Lactobacillus is linked by non-covalent intermolecular forces with the cell wall, in a spontaneous entropical process of reformation. Unlike the ample S-layer of gram positive bacteria, Lactobacillus inhibits repetition of the S-layer, causing it to be greatly diminished and highly predicted pl, promoting high concentration absorbance of Propidium Iodide. Despite having insufficient research on Lactobacillus S-layer it has been identified to play a major role in determining pathogenicity, ability to proliferate, integration in the gastrointestinal microbiota of mammals, the ability to adhere to host cells, or extracellular proteins, as well as facilitate enzyme expression and maintain cell structure. https://link.springer.com/article/10.1007/s00253-013-4962-2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4155827/
Peptidoglycan and Resistance
Because Lactobacillus is classified as a Gram-positive microbial strain, the protein peptidoglycan is the essential structural element to the cell wall. The peptidoglycan sacculus consists of a polymerizing chain of interchangeable N-acetylglucosamine, and N-acetylmuramic, binded by specific peptide bonds classified as 𝛃-1,4 peptide chains. This alternating peptide polymer chain is what gives the 3-dimensional structure of the bacteria cell and ensures Lactobacillus’ integrity. Lactobacillus incorporates an amino acid sequence of peptides that stem from the peptidoglycan sacculus, classified as carboxypeptidases, and endopeptidases, both active proteolytic enzymes responsible for the catabolism of amino acid peptide bonds. With an addition of D-Lac residues at the terminal ends of polysaccharide chains in the peptidoglycan layer of Lactobacillus is what prompts vancomycin-resistance of the microbe, allowing it to thrive in the gut microbiota, and also be classified to have pathogenic factors, labeling it as a nosocomial infective pathogen. https://www.sciencedirect.com/science/article/pii/S107455210000116
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4155827/
Peptidoglycan structure of Lactobacillus casei ATCC 393, a species highly resistant to glycopeptide antibiotics.
Allochthonous vs. Autochthonous?
Allochthonous and autochthonous bacterium are differentiated by their ecological niche and role in the host’s microbiome. In addition, allochthonous and autochthonous are a classification system to determine the colonization ability of a microbe in a microbiome. Identified to originate from fermented products, Lactobacillus is classified as nonnative inhabitants of the mammalian intestinal microbiome. Although Lactobacillus does not originate from the gut microbiome, it is capable of colonizing in high concentrations, adhering to the cilia surface of the epithelial lining forming resistant biofilms, and capable of populating in high concentrations. Steven A. Et Al identifies both an autochthonous and allochthonous strain of Lactobacilli, though autochthonous Lactobacillus strains are more efficient in proliferating in the gastrointestinal tract of human organisms. It has been identified that autochthonous strands of Lactobacillus are capable of increasing the mean corpuscular hemoglobin, increasing oxygen production and respiration of the gastrointestinal tract, promoting proper function of the digestive and respiratory system, and increasing glucose concentrations. Comparison of the Colonization Ability of Autochthonous and Allochthonous Strains of Lactobacilli in the Human Gastrointestinal Tract (scirp.org) Autochthonous vs allochthonous probiotic strains to Rhamdia quelen - PubMed (nih.gov)
Metabolism and Interaction with the Environment
General
Lactobacillus is a member of the lactic acid bacteria that synthesizes lactic acid as an end product of carbohydrate metabolism. Lactic acid bacteria (LAB), including Lactobacillus, have the capability of metabolizing polysaccharides and macromolecules present in synthetic foods, that the gut microbiota of mammalian organisms is incapable of breaking down. Located in the microbiome of the human gastrointestinal tract, Lactic acid bacteria act as a heterogeneous group of diverse taxa, playing diverse and distinct roles in the fermentation of indigestible polysaccharides. Lactic acid bacteria: their applications in foods - MedCrave online (https://www.frontiersin.org/articles/10.3389/fbioe.2021.612285/full) In addition, Lactic acid is capable of producing byproducts of short-chain fatty acids, amines, bacteriocins, vitamins, and exopolysaccharides during carbohydrate metabolism, other than lactic acid. The number of varying products formed by lactic acid bacteria is what allows it to be expansively used in the food industry, such as the flavor of fermented foods, reducing harmful microbes, and probiotics to improve the health of the gastrointestinal tract. The metabolism of Lactic acid bacteria is broken down into three different pathways, including the breakdown of sugars (glycolysis), breakdown of fat (lipolysis), and degradation of proteins (proteolysis). Lactic acid bacteria: their applications in foods - MedCrave online
Lactic acid bacteria are capable of hydrolyzing proteins, synthesizing viscous exopolysaccharides, and inhibiting bacterial proliferation of surrounding microbes, allowing them to be applied to biotechnological practices involved.
Metabolic pathway of Lactic acid bacteria of the homolytic and heterolytic fermentation of glucose into lactic acid, synthesizing electron carriers NADPH and ATP. Electron transport then reroutes products of glycolysis of pyruvate, producing more end products than lactic acid. https://www.frontiersin.org/articles/10.3389/fbioe.2021.612285/full
Polysaccharides are classified as polymer chains of monomers known as monosaccharides, binded by alpha or beta glycosidic bonds. https://www.frontiersin.org/articles/10.3389/fbioe.2021.612285/full#B63 The degradation of polysaccharides in synthetic foods by Lactobacillus allows the formation of different monosaccharides, including lactic acid (the monomer of lactose), improving the quality of food. Furthermore, probiotics, including Lactobacillus, is affected by the levels of polysaccharides present in the microbiome (saccharides that are classified as prebiotics). Lactic acid bacteria is also capable of transporting, degrading, and catalyzing proteins present in the gut microbiota. The metabolic pathways of Lactobacillus are then further divided based on chemical reactions initiated by protein expression in the bacteria This includes the expression of proteinase in the cell envelope that promotes the proteolysis in Lactobacillus, degrading proteins into oligopeptides, dipeptides, a polymer chain that consists of two amino acids, and tripeptide, triple amino acid polymers. Both amino acids are catalyzed and broken down, releasing energy for the microbial organism. https://www.frontiersin.org/articles/10.3389/fbioe.2021.612285/full
Prebiotics and Efficiency of Lactobacillus
Prebiotics are classified as organic, or nonorganic compounds that have chemical properties that promote the proliferation and efficiency of probiotical bacteria. Dietary fibers and inulin, are common examples that are anticarcinogenic compounds, enhancing the capability of Lactobacillus plantarum in inhibiting carcinogenesis of tumor cells. Prebiotics also are implemented as nutrients in the human intestinal tract, where Lactobacillus metabolizes complex carbohydrates that are usually non-binding by mammals' enzymes. Potential of probiotics, prebiotics and Synbiotics for management of colorectal cancer - PMC (nih.gov)
Prevention and Health
Overall, Cancer Impact
A key point highlighted in Effects of Probiotics, Prebiotics, and Synbiotics on Human Health, Paulina Markowiak and Katarzyna Slizewska, PubChem, stated the term Probiotic originated from Greek roots, meaning “for life.” The term was initially used by Ilya Ilyich Mechnikov in 1908 for fermented yogurt-derived products such as cheese, bread and wine, and their growth promoting medical properties. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5622781/ It has then been identified that probiotics are sourced from a varying degree of microbes including fungus and bacteria. Probiotics have been researched to treat several health disorders and diseases, such as inflammation, diarrhea, obesity, urogenital infections, but more recently, cancers. Probiotics, including Lactobacillus, have the capability of regulating cancer signaling. Making external changes to the cell cycle, including promotion of apoptosis of tumor cells, inhibition of mutagenic activity, or inhibition of oncogene expression. Probiotics promote the apoptosis of tumorous cells, initiating the fragmentation of DNA, reduction of cytoplasm and solutions in the cell, and prevention of lysis, by modulating specific expression of Bax/Bcl2, resulting the formation of small pores around the cell membrane, inducing the programmed death of the cell. Probiotics such as Lactobacillus acidophilus and Bifidobacterium bifidum have the ability of increasing the mRNA expression of hBD-2 genes inducing apoptosis of cancer cells, and promoting complete cell cycle arrest. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6566532/ These probiotic properties are based on the structure, metabolism, and cell communication expressed by the bacterial organism, also known as metabiotic. Metabiotics of probiotic organisms such as Lactobacillus can be divided based on the structural components, and metabolic components. Lactobacillus induces apoptosis of carcinogenic cells, acting as antimutagens preventing lethal mutations in cell DNA, and a reactivator of tumor suppressor reactivators.
Colorectal Cancer
In a primary research paper published by Naoki Sugimura, Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumorigenesis, metagenomic sequencing of Lactobacillus gallinarum in mice diagnosed with colorectal cancer, identifying a significant correlation between Lactobacillus populations in the gut microbiota and number of tumors present. Using mice as a model organism, Naoki Sugimura identified anticarcinogenic properties of Lactobacillus, reducing size significantly in the number of tumors in the intestine. They proved the existence of Lactobacilli’s carcinogenic properties by either injecting the mouse with MG1655 E. Coli, or Lactobacillus gallinarum, to identify the effects of microbiome bacteria as probiotics. Using methodological colonoscopies, they identified the state of the test subject's gastrointestinal tract and quantified the number of neoplastic lesions (lesions caused by abnormal cell growth.) Research concluded with identifying Lactobacillus’ possible future use as a probiotic in order to inhibit the synthesis of intestinal tumors and treat colorectal cancer. Lactobacillus has cytotoxic and anticarcinogenic effects on cervical, gastric, colon, melanoma, and breast cancer. Using mice as a model organism to study human biology, scientists were able to see the impact Lactobacillus has on activation/enhancing of immunal responses, as well as directly attacking cancer cells, and promoting arrest in the tumor cell cycle. Yingxue et. Al, Effects of Lactobacillus acidophilus KLDS1.0901 on Proliferation and Apoptosis of Colon Cancer Cells, overviews the cytotoxicity effect of lactic acid bacteria, that promotes the apoptosis of colon cancer cells, reducing expression of HT-29 and Caco-2 cells, reducing expression of the mitochondrial membrane potential of HT-29 tumor cells, and promoting signaling pathways responsible for cancer cell apoptosis. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6775487/ https://gut.bmj.com/content/early/2021/12/21/gutjnl-2020-323951 Zackular et. al studied treatments of mice by administering the carcinogen azoxymethane, to promote chemically induced colon cancer in order to identify the effects Lactobacillus has on proliferation of tumor masses in the colon, and inhibition of precancerous growths. Lactobacillus is shown to limit the presence of carcinogenic causing microbes such as Bacteroides fragilis and Escherichia coli, shifting the presence of tumorigenesis in the gut microbiome. The Gut Microbiome Modulates Colon Tumorigenesis - PMC (nih.gov)
Effect of Lactobacillus acidophilus Dietary Supplements on 1,2-Dimethylhydrazine Dihydrochloride-Induced Intestinal Cancer in Rats23 | JNCI: Journal of the National Cancer Institute | Oxford Academic (oup.com)
Lactobacillus gallinarum defends against tumorigenesis in Apcmin/+ mice, depicting a timeline and colonoscopy of each math subject. Both male and female mice have a control non-treated of probiotic Lactobacillus.
Cervical Cancer
Cervical cancer is the fourth leading malignant cancer diagnosed among women worldwide. Alterations of the vaginal microbiota leads to a multitude of gynecological diseases including cervical carcinogenesis. Probiotics, including Lactobacillus Crispatus, Lactobacillus jensenii, and Lactobacillus gasseri has shown to prevent the expression of CDK2, cyclin A, and HPV oncogenes, responsible for cervical cancer, and promoted anticancer activities against specifically HeLa cervical cancer. Lactobacillus also is capable of promoting the upregulation of apoptotic genes, including caspase3, caspase8, caspase9, BAX and BAD, promoting the programmed cell death of tumor cells, and acting as an anti-carcinogen. Role of Lactobacillus in cervical cancer | CMAR (dovepress.com) Lactobacillus and the metabolites produced, such as lactic acid, is capable of preventing proliferation and horizontal gene transfer of invasive pathogens adhering to the epithelial lining of cervix, preventing the induction of malignant tumor formation, and promoting anti tumor suppression of the immune system, such as natural killer cells, dendritic cells and T cells. Role of Lactobacillus in cervical cancer - PMC (nih.gov) According to the case study, Wang et. Al Inhibitory effect of vaginal Lactobacillus supernatants on cervical Cancer cells (2018), 3 strains of Lactobacillus (crispatus, jensenii, gasseria), has the capability of inhibiting the growth of Caski cells, altering its physical structure, decreasing the number of tumor cells present that lack tumor suppressors, and expression of oncogenes. Furthermore, Lactobacillus has the ability to mute expression of CDL2 and Cyclin A, decreasing the overall expression of HPV oncogenes, and decreasing the number of tumor cells.
Inhibitory Effect of Vaginal Lactobacillus Supernatants on Cervical Cancer Cells - PubMed (nih.gov)
Breast Cancer
Lactic acid Bacteria, as a result of inhibiting the gastrointestinal microbiome, and systemic immune system of mammalian organisms, has the potential to limit breast cancer formation. Gastrointestinal microbes have a significant impact on the circulation and regulation of estrogen levels through the release of β-glucuronidase, a molecule responsible for the deconjugation of estrogen into a form capable of being absorbed by tissue. This increased absorbance is a major cause of carcinogenic malignancies. such as breast cancer. Potential effect of probiotics in the treatment of breast cancer - PMC (nih.gov) Long-term administration of the lactic acid bacteria strain: Lactobacillus plantarum, has shown effective anticarcinogenic effects, limiting the amount of tumor necrosis factors alpha in breast cancer, and increasing the number of CD4+ T-cells to combat carcinogenic infections. With the use of the carcinogen N-nitroso-N-methylurea, a group of scientists induced breast cancer in mice test subjects to test the effectiveness of lactobacillus as a probiotic anti-carcinogen. The study concluded that administration of the Lactobacillus strain casei, promotes the production of Th1 cytokine production in the spleen culture of mice. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy - PMC (nih.gov) Soy products enriched with Lactic acid bacteria are known to have a positive impact preventing breast cancer, decreasing the risk of patients developing such malignancies. In the study of Chiaki. Et. Al Lactobacillus casei Shirota enhances the preventive efficacy of soymilk in chemically induced breast cancer, a study located in Japanese, where women were exposed to 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine at 85 mg/kg bodyweight, were consistently monitored for estrogen levels. Compared to a control group, women who ingested soymilk with a combination of Lactobacillus casei, had a decrease in the development and volume of mammary tumors. Lactobacillus casei Shirota enhances the preventive efficacy of soymilk in chemically induced breast cancer - PubMed (nih.gov)
Impact on Gut Brain Axis and Alzheimer's
The gut-microbiota has been identified to moderate brain function and expression, through an interconnected signal system known as the gut-brain axis. Furthermore, it has been determined inflammation in the gut has a direct correlation with the neurodegeneration of neurons in the brain, including neurodegenerative disorders such as Alzheimer's. The gut-brain axis is a bidirectional signal mainly transmitted from the enteric and central nervous system through the vagal nerve through several different neurotransmitters such as serotonin, dopamine, Gamma aminobutyric acid (GABA), and glutamate. Each neurotransmitter can be enhanced through expression of probiotics. Because of Lactobacillus’ beneficial impact on the regulation of neurological inflammation of the gut, and inhibition of damage to the gut microbiome of mammalian organisms, and gut brain axis, it has been researched how Lactobacillus as a probiotic supplement could be applied to alter the stability gut brain axis and help contribute to treatments for cognitive disorders such as Alzheimer's. Alzheimer's is a progressive chronic condition that is caused by A𝛃 plagues, inducing neuron damage and loss. Different treatments for Alzheimer's focus on promoting tramiprosate and ALZ-801, that inhibit the synthesis of A𝛃 plagues. It also has been shown that a loss of biodiversity in the gastrointestinal tract, can lead to the development of Alzheimer's disease, altering the homeostasis of the brain's neurotransmitters and damage of the gut brain axis. Impairs in the gastrointestinal epithelial layer induce inflammation of neurons in the gut brain axis and accelerate Alzheimer's. Lactobacillus suppresses inflammation of the neurons through promotion of the synthesis of indole-3-aldehyde, and indole-3-propionic acid, which is transferred through the brain blood-barrier. https://microbiologyjournal.org/impact-of-gut-microbiome-lactobacillus-spp-in-brain-function-and-its-medicament-towards-alzheimers-disease-pathogenesis/ https://onlinelibrary.wiley.com/doi/10.1002/ame2.12033
Autism and Lactobacillus
The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders, a primary report published by Basma Abdellatif, reported a distinct correlation between the gastrointestinal gut brain axis and patients that display traits on the Autism spectrum disorder. Autism spectrum disorder is a condition that appears to affect 0.1% to 1.8% of the population, caused by epigenetic, genetic, and environmental components that impact its prevalence in the host. External factors include maternal exposure to virus, deficiency or overload in nutrition in embryo stage, and a dysfunctional immune system. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7312735/ It has been identified that probiotics including lactic acid bacteria is capable of promoting the hosts health, stabilizing the gut’s microbiota, and restoring the balance of microbial diversity, furthermore optimizing the gut-brain axis, and gastrointestinal barrier. The neurological connection between gut and brain is a cooperative neurological pathway, whereupon the gut impacts neurological activation in the brain, and the brain impacts neurological signals of the gut microbiota. Impacts the brain has on the gut microbiome includes the amount of blood reaching the gastrointestinal system, its motility, and permeability. The gut microbiome on the other hand can influence the neurological system, by excreting metabolites that alter the health of the host, such as anti-inflammatories for inflamed or damaged neurons, as well as nutrition for the epithelial cell lining of the colon. Lactobacillus plantarum PS128 and Other Probiotics in Children and Adolescents with Autism Spectrum Disorder: A Real-World Experience, published by Martina Maria Mensi, observed the probiotic Lactobacillus plantarum is effective in the symptom management of Autism Spectrum Disorder, lessening expression of symptoms of the disorder, reducing body weight, as well as improvement in Autism expressed traits by the individual. https://pubmed.ncbi.nlm.nih.gov/34198499/ https://pubmed.ncbi.nlm.nih.gov/28686541/
Conclusion
Lactobacillus as a probiotic maintains a healthy gut microbe flora by breaking down nutrients and polysaccharides that are naturally incapable of being digested by the mammalian native microbiome. Additionally, Lactobacillus can be used as probiotics in carcinogen treatment, medicating a diverse range of cancers, including colorectal, cervical, and breast cancer. With a direct impact to the gut brain axis, Lactobacillus is able to excrete probiotics that regulate the inflammation of neurons critical for signals that are sent to the brain, altering neurological system pathways, and preventing damage of neurons that promote diseases such as Alzheimer's and Parkinson's.
References
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College