Sjögren Syndrome
Section
By Ryan Yarcusko
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC. Every image requires a link to the source.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Sample citations: [1]
[2]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
To repeat the citation for other statements, the reference needs to have a names: "<ref name=aa>"
The repeated citation works like this, with a forward slash.[1]
Introduction
Sjögren’s syndrome is a human autoimmune disease affecting 4 million Americans today, which causes disruption in the exocrine glands [1][2](Mayo, Rhodes). Salivary and lacrimal glands are among the most adversely affected, which leads to characteristic symptoms such as dry mouth (xerostomia) and dry eyes (keratoconjunctivitis sicca, or KCS) (Trujillo). In turn, xerostomia and KCS lead to high susceptibility to opportunistic pathogens such as Candida albicans and Pseudomonas aeruginosa (Mun, Anil). Until recently, limited public awareness has prevented focused research and treatments efforts (Rhodes).
History
The first known documented case of Sjögren’s syndrome was in 1888 in London, England. It is theorized that the 65 year old woman had Sjögren’s syndrome because she presented with dry eyes and xerostomia (Parke). As these cases became more prevalent in the early 1900s, Johann von Mikulicz-Radecki determined that any patient with enlarged salivary and lacrimal glands would be diagnosed with Mikulicz’s disease. However, there are many different disorders that present with enlarged salivary and lacrimal glands, so only patients with chronic inflammation of these exocrine glands were diagnosed with Miculicz’s disease (Parke). After this change, incidences of patients with the specific subset of symptoms associated with Sjögren’s syndrome were documented, but the symptoms were not characterized until 1930 (Parke).
In 1930, a Swedish ophthalmologist by the name of Henrik Sjögren performed a study on a series of patients presenting with these symptoms (Fig. 1). Ultimately, Sjögren published a thesis describing the characteristics of the disease in 19 females, describing the syndrome as “keratoconjuctivitis sicca” (Parke). Interestingly, during this time, many other physicians had written and published papers describing this unique set of symptoms, but Sjögren had the most widely accepted thesis. Additionally, there was widespread confusion regarding the difference between Mikulicz’s disease and Sjögren’s syndrome, but it was ultimately decided that the two conditions are the same. Because of this, the syndrome carries Sjögren’s namesake still today (Parke).
Overall, the history of Sjögren’s syndrome can be divided into three prominent subsections. First, the basic characterization of the symptoms of Sjögren’s syndrome. Next, the mechanistic characterization of the associated immune system phenomena. Finally, beginning in the 1980s, a deeper understanding of the overall process of the disease and related symptoms through the expanding field of molecular biology (Parke).
Symptoms
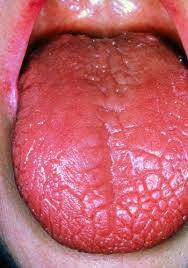
Inflamed lacrimal and salivary glands are the most common symptoms of Sjögren’s syndrome (Mayo). This, in turn, can create a wide variety of secondary symptoms. The inflammation of lacrimal and salivary glands prevents the lubrication of the mouth and eyes, which gives rise to KCS and xerostomia (Mayo). This dryness can be extreme, in some cases causing the tongue to look like crocodile skin (Fig. 2) (Parke). Other prominent symptoms which result from limited bodily lubrication include vaginal dryness, dry skin, skin rashes, and persistent dry cough (Mayo).
Additionally, symptoms resulting from a weakened immune system include joint pain and fatigue (Mayo). A weak immune system causes a general weakening of the body, which has been found to damage multiple tissues in the human body, including the kidneys, thyroid, liver, lungs, and nerves (Mayo).
Causes
Sjögren’s syndrome is an autoimmune disorder where the immune system mistakenly attacks the body’s own cells and tissues (Mayo). This can occur in many different places in the body, but in Sjögren’s syndrome, the body’s white blood cells most frequently attack the salivary and lacrimal glands in a process known as lymphocytic infiltration (Tsubota) (Fig. 3). Lymphocytic infiltration is what causes the inflammation of these two exocrine glands.
The specific cause of lymphocytic infiltration remains unclear, but there are some factors associated with a high incidence of disease. For example, some genes have been shown to increase susceptibility to Sjögren’s (Cobb). While the research efforts regarding the causes of Sjögren’s remain minimal, it has been determined that a triggering event such as viral or bacterial infection is necessary for disease onset (Mayo). Some studies have found that patients have a skewed B cell maturation following the influenza vaccine, and thus an improperly formed immune system (Wiggins). Falling levels of estrogen have also been found to trigger infection (Gieffen). A subset of Sjögren’s syndrome, known as secondary Sjögren’s syndrome, is triggered by the onset of some tissue diseases, such as lupus, which also arises from lymphocytic infiltration (Mayo). This is distinct from primary Sjögren’s syndrome, which is not triggered by another disease.
Diagnosis
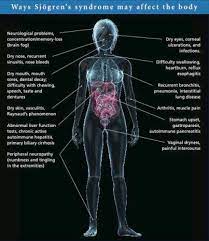
Clinical diagnosis of Sjögren’s syndrome can be difficult because notable symptoms can span the entirety of the human body (Fig. 4). Additionally, symptoms can be highly variable between individual patients (Mayo). Because of this, patients may need to see different physicians for different symptoms. In fact, it takes an average of 3.9 years after onset for a patient to be diagnosed with Sjögren’s syndrome (Rhodes).
To make diagnosis easier, clinicians have developed a series of medical tests that can narrow the possibilities down. For example, blood tests may reveal characteristic signs of Sjögren’s syndrome. Firstly, Sjögren’s syndrome antibodies may be found in the blood (Mayo). Secondly, molecular evidence of inflammatory conditions, such as kidney and liver damage, may be detected in a blood test (Mayo). When the liver is damaged, enzymes leak from the liver, which causes abnormally high liver enzymes to be found in the bloodstream (Iwasaki). A functioning Kidney will eliminate blood urea nitrogen (BUN) and creatine from the kidneys. If these molecules are in high concentration in the bloodstream, there has likely been kidney failure, which can be a symptom of Sjögren’s syndrome.
Ophthalmologists may also check for signs of Sjögren’s in the eye. A slit lamp uses a bright beam of light with a focal lens to examine various layers of the eye (Martonyi). A slit lamp can be used to check for corneal damage, which results from eye dryness (Mayo). Additionally, ophthalmologists may use a Schirmer tear test, where a small piece of filter paper is placed under the eyelid (Mayo). The amount of moisture on the filter paper after a given time may be used to measure eye dryness.
In the mouth, physicians can use a sialogram test. In the sialogram test, a colorful dye is injected into the salivary glands to determine the quantity of saliva that is entering the mouth (Mayo). Another interesting clinical test is salivary scintigraphy, which involves the injection of a small amount of radioactive element, technetium 99, into the patient’s vein. The location of this radioactive element can be tracked over an hour to determine both how it moves through the body, and how long it takes to arrive at the salivary glands (salivary glands website). This test can also be used to scan for the size and shape of the inflamed salivary glands. Finally, some physicians use a biopsy to remove portions of the salivary glands in order to examine them for inflammatory cells that are characteristic of Sjögren’s syndrome (Mayo).
Treatment
Short-term, non-invasive treatments for Sjögren’s syndrome include hydrating eye drops and increased water intake to combat xerostomia and KCS (Mayo).
Many secondary symptoms can also be treated with drugs. Xerostomia and KCS can be prevented by pilocarpine (Salagen) and cevimeline (Exovac), which increase the production of saliva and tears. Because these drugs increase fluid output, this can lead to side effects such as excessive sweating and urinary and gastrointestinal disturbances (Fox). Yeast infections in the mouth can be treated with antifungal medications (CDC). Arthritis symptoms may be treated with typical arthritis medicines, including celecoxib, nabumetone, and piroxicam (Mayo). Drugs that suppress immune system response may also be helpful, because they limit the severity of the immune system’s attack on the body.
Physicians have also developed more invasive options for the treatment of Sjögren’s syndrome. For example, collagen or silicon pills have been inserted into the tear ducts of the eye to prevent tear drainage, which allows the eye to retain moisture (Mayo). The foundations for the creation of artificial salivary glands with tissue engineering are also being laid (Aframain).
Although minimal progress has been made, novel treatments are under development for Sjögren’s syndrome. One study induced B-cell depletion in a patient with primary Sjögren’s using rituximab. Rapidly after B-cell depletion in the patient, remarkable improvement in xerostomia occurred (Ring, Pijpe). Another interesting mechanism of treatment is interferon (INF) therapy. INFs are proteins with a wide range of regulatory functions including cell proliferation and differentiation, enzyme induction, and cell surface antigen expression (von). One study has shown that an INF lozenge taken orally by the patient can increase saliva secretion (Khurshudian). Preliminary gene therapies have also shown promise for curing Sjögren’s by altering the DNA of the patient (Baum).
Risk Factors
Women are at high risk of Sjögren’s syndrome. In fact, women are as much as 10 times more likely to develop this disease than men (Johns Hopkins). Middle aged people are also more likely to develop Sjögren’s, specifically patients aged 40-60 years old (Johns Hopkins, Mayo). In contrast, this disease is exceedingly rare in children (Bartunkova). Rheumatic diseases such as rheumatoid arthritis are also risk factors which pose a threat of causing secondary Sjögren’s (Mayo).
Complications
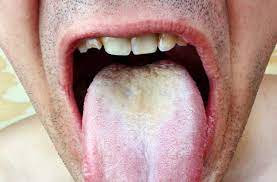
The vast variety of symptoms of Sjögren’s leads to a long list of medical complications. As the mouth dries out, the typical microbial and molecular environment is not maintained, which causes a weakening of the teeth, and cavity formation (Mayo, Parke). Similarly, oral thrush (Fig. 5) and yeast infection may occur due to a lack of microbial defenses (Parke). In fact, secondary bacterial infection can occur in any place that is dryer than normal in the body (Mayo). Smokers may face more severe symptoms because smoking cigarettes can further dry out the mouth, which compounds the issues resulting from xerostomia (Thomson). KCS can also make patients more susceptible to corneal damage, light sensitivity, and blurred vision (Iyer, Koh).
In general, inflammation of the immune system has the potential to cause problems with many bodily systems. Lung problems, such as pneumonia, are not uncommon (Mayo). Hepatitis in the liver and limited kidney function are also common in patients with Sjögren’s. Although less common, some patients with Sjögren’s have developed cancer in the lymph nodes (Lai). Some patients have also developed peripheral neuropathy (Koike).
Microbial Consequences
Yeast Infection
Under normal, healthy conditions, the vagina is typically lubricated with a matrix containing protective agents such as white blood cells (Robboy). In addition, the vaginal microbiome has been theorized to create a biological barrier to pathogenic microorganisms (De Seta). Lactobacilli are the most common microbe isolated from the healthy vaginal microbiome (Hummelen) (Fig. 6). In fact, Lactobacilli are known to use a variety of mechanisms to inhibit the growth of pathogens (Gregor). Lactobacilli secrete anti-adhesion factors to prevent the adhesion of other microbes to the surface of the vagina. Lactobacilli also produce byproducts such as hydrogen peroxide and bacteriocins that work as antibiotics against other pathogenic bacteria. Finally, Lactobacilli are thought to stimulate the body’s immune system response, upregulating the body’s natural antimicrobial agents in the vaginal microbiome.
Due to the decreased secretion rate of exocrine glands, Sjögren’s syndrome is known to cause vaginal dryness (Nimwegen), which results in a lowering of antimicrobial defenses. Firstly, decreased lubrication of the vagina means that there will be decreased flow of antimicrobial agents within the vaginal microbiome (Robboy). Secondly, microorganisms that occupy the vaginal microbiome may not be fit to live in dry environments, so important defenders will die off, leaving the vagina more susceptible to pathogenic attack (De Seta).
This susceptibility to pathogenic attack is readily apparent from a variety of clinical studies. In female patients with primary Sjögren’s syndrome, cervicitis (inflamed tissue in the cervix) has been observed with incidence rates as high as 42% (Capriello). However, one of the most common vaginal diseases associated with Sjögren’s is yeast infection. Candida is a species of opportunistic, pathogenic fungus that tends to live in various locations in the body (Berman). Candida (most commonly Candida albicans) are often isolated from the vaginal microbiome of healthy patients, and infection will not occur until after some causative agent occurs, such as a weakened immune system (Vazques). When the vaginal microbiome dries out, the lower concentration of human defense molecules and the decreased diversity of native microbes often lead to higher rates of candidiasis, or yeast infection (Bedford).
Aside from having a higher tolerance for a range of biological conditions, Candida albicans is able to outcompete native microorganisms through an interesting mechanism known as morphological transition (Vazques). Through this process, Candida albicans are able to change morphologically from blastoconidia to pseudohyphae and hyphae. This morphological transition is created in response to environmental conditions. This extreme form of adaptation is considered one of the most important factors in pathogenicity of Candida albicans.
Dry Eyes (keratoconjunctivitis sicca)
In comparison to the oral and vaginal microbiome, the ocular microbiome has been found to contain an exceedingly low number of microbial organisms (Perkins). Conjunctival cultures done on the eye have found relatively inconsistent concentrations of specific microbes between patients, but among the most commonly found microorganisms are Staphylococcus epidermidis and Propionibacterium acnes. However, some ocular bacteria are not culturable due to specific growth requirements, so metagenomic analysis has been done to characterize the ocular microbiome (Schabereiter). Metagenomic approaches have found similar results, with bacteria being present in much lower quantities in comparison to other bodily surfaces (Doan). It is also important to note that, due to the low concentration of ocular microbes, DNA from microbes that cannot persist on the ocular surface can skew the results of metagenomic studies. In such a case, metagenomic characterization may suggest that there are microbes living on the eye, even if they have simply lysed (Rosseel). Regardless, there is not any one species of bacteria that is found in all or most ocular microbiomes (Ozkan).
Although there is not a general, characteristic ocular microbiome of a healthy individual, some studies have shown that the bacterial microbiome can protect the eye from exogenous pathogens (Kugadas). For example, studies have found that commensal molecules from bacteria inhabiting the ocular microbiome may react with antigens on the surface of the eye to encourage antibiotic production (Trujillo). To compound these microbial defense mechanisms, when the lacrimal glands secrete into the eyes, the secretion contains antibiotics and a slew of other molecules to protect the eye from pathogens (Mun).
As the eye dries out from Sjögren’s syndrome, and the lacrimal glands do not secrete sufficiently, the microbial significance is twofold. Firstly, KCS can kill off the few commensal bacteria that typically live on the surface of the eye. Without the native bacteria, the body’s immune response will not be stimulated, and thus, there will be less antibiotic production (Trujillo). More importantly, the lacrimal glands will be unable to secrete the fluid that contains the antibodies. As a result, there is a strong correlation between KCS and pathogenic susceptibility. In studies comparing the microbiome between patients with and without KCS, higher numbers of colony-forming bacteria have been found on the ocular surface of patients with KCS (Graham). Additionally, increased microbial diversity on the ocular surface has been shown to increase with severity of ocular disease (Shimizu). An additional secondary consequence of KCS is that increased damage can occur. This results in higher incidences of corneal tears, which create an opening in the body’s ocular surface, allowing pathogenic bacteria to enter deeper into the eye tissue (Metruccio).
Of the bacteria that use pathogenic pathways to infect the eye, Pseudomonas aeruginosa (Fig. 7) is the most prevalent, attacking through the cornea (Mun). When healthy antimicrobial defenses are weakened, Pseudomonas aeruginosa will release enzymes and toxins which degrade the cornea, causing a condition known as bacterial keratitis (Kreger). In bacterial keratitis, the patient experiences eye pain, reduced vision, and light sensitivity (Mascarenhas). Much of the Pseudomonas aeruginosa pathogenic success can be attributed to its high acquisition of antibiotic resistance genes (Subedi). Pseudomonas aeruginosa acquires antibiotic resistance genes through integrons and transposons. In Sjögren’s, the decreased antibiotic concentration on the surface of the eye combined with the high antibiotic resistance of Pseudomonas aeruginosa makes pathogenetic infection of the eye much more likely.
Dry Mouth (xerostomia)
The delicate balance of the oral cavity is relatively complex. In a healthy patient, the salivary glands are necessary for maintaining oral homeostasis, function, and health (Anil). Aside from secreting enzymes for food digestion, the salivary glands are also responsible for lubricating the oral cavity to reduce friction during chewing and talking. Thus, patients with xerostomia are more likely to suffer from mouth injuries due to high friction. These openings in the dermis of the oral cavity can present opportunistic entry points for pathogenic bacteria to infect the mouth (Anil). Xerostomia can also lead to food retention in the mouth, which offers a rich nutrient source which is not normally present in the oral cavity. This can select for invasive bacteria that otherwise would be unable to outcompete the native microorganisms (Anil).
In addition to lubrication, the matrix secreted by the salivary glands contains electrolytes, peptides, glycoproteins, enzymes, immunoglobulin A, amines, and leukocytes, which select for the native microorganisms in the oral cavity, and select against pathogenic microbes (Anil). Most native microflora in the mouth are incredibly important to pathogen resistance in humans. For the most part, these microbes are responsible for stimulating the immune response in the oral cavity, which is then upregulated to combat pathogens (Ruby).
Unfortunately, some native microorganisms can be harmful to oral health under the right conditions. Streptococcus mutans and Streptococcus sobrinus, for example, live in the plaque on teeth. These lactic acid bacteria secrete extracellular glucans which form a less dense matrix (plaque). The plaque allows sucrose to travel through, which permits the production of lactic acid via fermentation (Liljemark). Typically, as long as good oral hygiene is maintained, this is not an issue. However, saliva production is extremely important for diluting the concentration of the lactic acid produced by these bacteria (Ruby). When a patient has Sjögren’s, the minimal salivary gland secretion leads to a high concentration of lactic acid (Ruby).
The high concentration of lactic acid within the mouth often leads to the breakdown of teeth (Parke). This breakdown can progress to cause cavities and complete loss of teeth. As teeth are lost, the dental pulp, which is a vascularized connective tissue lined with odontoblasts, is housed within the tooth, and is highly susceptible to bacterial infection (Ruby). Dental pulp exposed to the bacteria of the mouth is extremely likely to become seriously infected.
Additionally, The bacteria that produce this lactic acid are able to tolerate the high acidity levels, but much of the oral microbiome cannot. As the native microbiome is weakened by acidic conditions, some opportunistic pathogens are able to outcompete the other oral microbes, causing infection in the host (Ruby). Candida albicans is a normal inhabitant of a healthy oral microbiome (Mayer, Ruby) (Fig. 8). Because Candida albicans is able to live in low pH environments, it can effectively colonize the mouth, leading to a disease known as thrush (Ji, Anil). This results in symptoms such as redness, burning, and difficulty swallowing. In immunocompromised patients, such as those with Sjögren’s, the infection can be much more serious (Mayer).
Public Awareness
Despite the severity and consequences of Sjögren’s syndrome, public awareness has remained low for many years. Although Henrik Sjögren wrote his thesis on Sjögren’s syndrome in 1930, it was not translated from German to English until 1943 (Parke). In some aspects, this lag in awareness has persisted even today, even for the physicians that diagnose Sjögren’s syndrome. For example, scientists were able to construct a computer-assisted statistical method for use by physicians to determine whether a patient has Sjögren’s syndrome, but, despite reducing costs in time and money for diagnosing a condition that affects 4 million Americans, the method has still not been adopted by ophthalmologists (Rhodes, Anderson).
More recently, in 2014, well-known tennis star Venus Williams came forward about her battle with Sjögren’s syndrome (Rhodes) (Fig. 9). This allowed awareness of the disease to gain substantial public traction. Notably, the Sjögren’s Syndrome Foundation (SSF) has made incredible leaps for people with Sjögren’s, leading advocacy and awareness campaigns to educate the general population about Sjögren’s syndrome. In fact, the SSF created world Sjögren’s day, which occurs every year on July 23rd, the birthday of Henrik Sjögren.
References
[1] Mayo Clinic Staff. “Sjogren's Syndrome.” Mayo Clinic, Mayo Foundation for Medical Education and Research, 2 Aug. 2022, https://www.mayoclinic.org/diseases-conditions/sjogrens-syndrome/symptoms-causes/syc-20353216#:~:text=Overview,as%20rheumatoid%20arthritis%20and%20lupus. [2]Rhodes, Ronale Tucker. "Sjögren’s Syndrome."
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2023, Kenyon College