Flooded Soils
Introduction
Flooded soils occur with complete water saturation of soil pores, and generally result in anoxic conditions of the soil environment. Flooded soil environments may include such ecosystems as: rice paddies, wetlands (swamps, marshes, bogs), compacted soils, and post-rain soils. Additionally, similar redox conditions (where oxygen is lacking) can also be found within soil aggregates and along pollutant plumes, and thus many of the concepts discussed in this section may be applied to those environments as well.
Oxygen is only sparingly soluble in water and diffuses much more slowly through water than through air. What little oxygen that is present in saturated soils in the form of dissolved O2 is quickly consumed through metabolic processes. Oxygen is used as terminal electron acceptor via respiration by roots, soil microbes, and soil organisms (Sylvia, 2005), and is lost from the soil system in the form of carbon dioxide (CO2). Heterotrophic respiration may completely dissolve oxygen in flooded soils; and these effects may be observed within only a few millimeters of the soil surface (Schlesinger, 1996).
Due to the deficiency of oxygen in flooded soils, those organisms inhabiting flooded soils must be able to survive with little or no oxygen. Although energy yields are much greater with oxygen than with any other terminal electron acceptor (see electron tower theory, section 2.1.1), under anoxic conditions anaerobic and facultative microbes can use alternative electron acceptors such as nitrate, ferric, manganese (IV) oxide, sulfate, and carbon dioxide to produce energy and build biomass.
Processes
In general, flooded soil condition occurs due to seasonal flooding or agricultural activity. The flooded soils condition can be often converted into non-flooded soil condition by the water level fluctuation and drainage. Through this variation of soil condition, various gases are emitted into the atmosphere or environmental factors, such as redox potential (Eh), pH, acidity, alkalinity, and salinity, are continuously changed. As explained in the introduction, microorganism can use alternative terminal electron acceptor when dissolved oxygen is absent. They successively use electron acceptor according to the order of electron acceptor utilization based on electron tower. The order change of electron acceptor utilization is observed in soil aggregates and pollutant plume. (Figure aggregates and pollutant plume)
Oxidation/reduction (redox) reaction
Electron tower
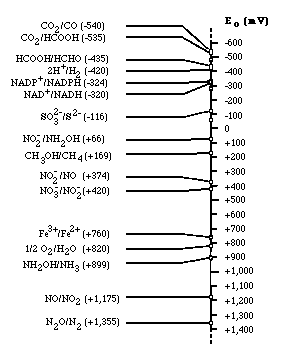 ,
http://www.microbiologybytes.com/introduction/Environmental.html
Electron tower theory explains the utilization order of electron acceptor for respiration.
Depending on the type of electron acceptors used by microorganisms, microbes can be classified into the strict aerobes, obligate anaerobes, and facultative anaerobes. The strict aerobes can not live under anoxic condition; on the contrary, obligate anaerobes can never use oxygen as electron acceptor. However, facultative anaerobes can live in both aerobic and anaerobic condition. If oxygen is plentiful, they tend to use oxygen because microorganisms gain much energy from reducing oxygen rather than other electron acceptors. When there is no more available oxygen in solution, they start to use nitrate as electron acceptor. Thus, obligate anaerobes and facultative anaerobes use alternative electron acceptor in the order of electron acceptor having more reducing energy. Oxygen is most efficient electron acceptor, while carbon dioxide has the less reducing energy.
,
http://www.microbiologybytes.com/introduction/Environmental.html
Electron tower theory explains the utilization order of electron acceptor for respiration.
Depending on the type of electron acceptors used by microorganisms, microbes can be classified into the strict aerobes, obligate anaerobes, and facultative anaerobes. The strict aerobes can not live under anoxic condition; on the contrary, obligate anaerobes can never use oxygen as electron acceptor. However, facultative anaerobes can live in both aerobic and anaerobic condition. If oxygen is plentiful, they tend to use oxygen because microorganisms gain much energy from reducing oxygen rather than other electron acceptors. When there is no more available oxygen in solution, they start to use nitrate as electron acceptor. Thus, obligate anaerobes and facultative anaerobes use alternative electron acceptor in the order of electron acceptor having more reducing energy. Oxygen is most efficient electron acceptor, while carbon dioxide has the less reducing energy.
Variation of pH and Eh
pH
Neutral pH soil
When soil is saturated with water, pH drops at first due to organic acid produced from fermentation. Then, pH gradually starts to rise because H+ is consumed via respiration of the aerobes and anaerobes. The half reactions of hydrogen consumption are as follow;
Aerobic respiration: ½ O2 + 2e- + 2H+ -> H2O (by facultative anaerobe and aerobes)
Iron reduction: Fe(OH)3 + 3 H+ + 2e- -> Fe2+ + 2H2O (by Iron reducing bacteria)
Denitrification: 2NO3- + 12 H+ +10e- -> N2+6H2O (by Denitrifier)
Sulfate reduction: SO42- + 10H+ +8e- -> H2S + 4H2O (by sulfate reducing bacteria)
Methane production: CO2 + 8 H+ + 8e- -> CH4 +2 H2O (by Methanogeous)
Manganese reduction: MnO2 + 4H+ + 2e- ->Mn2+ + 2H2O (by Manganese reducing bacteria)
Eh
During the succetion of anaerobic oxidation processes, the redox potential (Eh) of the flooded soil will decrease as a result of the reduced products formed. The approximate redox potential values that indicate the start and end of a specific reduction oxidation process are as follows: (How to make a table??) Observation Eh (mV) Disappearance of oxygen +330 Disappearance of nitrate +220 Appearance of manganese ions +200 Appearance of ferrous ions +120 Disappearance of sulfate -150 Appearance of methane -250
Solubility/mobility of mineral
Since the toxicity, solubility, mobility,and bioavailability of a given element or compounds are mainly influenced by soil solution redox potenial and pH, flooded soil condition plays an important role in mobility of trace metal, nutrients, and mineral.
Effects on life
In flooded conditions microorganisms can no longer use oxygen, or it is severly limited
Plant nutrient availability
Flooded soils can prevent efficient gas exchange between the plant root and the soil. pH plays a main role in a healthy plant growth process. In flooded soils, under anaerobic conditions the pH value wil tend to rise. Denitrification of soil nitrate to nitrogen gas plays a major role in the rise of pH levels. Flooding results in poor soil aeration because the supply of oxygen to flooded soil is severely limited. Oxygen deficiency is likely the most important environmental factor that triggers growth inhibition and injury in flooded plants. Generally speaking, the micro-nutrients (Fe, Zn, Mn, Cu) get locked out at a high pH (alkaline) above 7.0, while the major nutrients (N, P, K, Mg) can be less available in acidic soil (below 5.0).
In soil gardens signs of over watering include: Leaf wilting/drooping and Chlorosis (Leaf Yellowing). Also, smelly soggy soil is another indication in soil gardens. Solution - Increase the temperature and airflow to evaporate some of the excess water. Also, you can add some h2o2 when watering to help the roots still receive O2. And just don’t water as much. You should only water when your soil/medium is dry. If you have smelly soggy soil the best thing to do is transplant it into fresh dry soil.
What over watering looks like
Microorganism activity
In anaerobic respiration oxygen is replaced by other compounds as TEA (terminal electron acceptors). Iron is reduced from Fe3+ to Fe2+ during iron repiration. Manganese is also reduced. These processes occur because of microoganism activity. Energy yields are lower than for aerobic respiration The electron tower is used to determine amount of energy available from any pair of redox reactions.(1) Since oxygen is at the bottom of the electron tower(see above), it will yield the most energy when used as a TEA. But in flooded anaerobic condirtions, microorganisms will have to use other compounds.
Key Microorganisms & exchanges of gases
The role of microorganisms under flooded soil
nitrate reducing bacteria
Denitrification is carried out by obligate respiratory bacteria belonging to the genra Agrobactterium, Alcaligenes, Bacillus, Paracoccus, Pesudomonas and Thiobacillus (Knowles, 1982). Nitrate ammonification found in facultative anaerobe bacteria belonging to the genera Bacillus, Citrobacter and Aeromonas, or in the memebers of the Enterobacteriaceae (Cole adn Brown, 1980; Smith adn Zimmerman, 1981; MacFarlane and Herbert, 1982). Strictly anaerobic bacteria belonging to the genus Clostridium are also able to reduce nitrate to ammonia (Hasan and Hall, 1975). Pure culture studies show evidance that nitrate reduction does occure in presence of oxygen (Kuenen and and Robertson, 1987).
Methaneous bacteria
Methanogen (e.g Methanobacterium formicum, Methanobacterium bryantii, Methanobacterium thermo-autrotrophicum, and etc ) can use CO2 and produce methane (Langston and Bebiano 1998)
Iron reducing bacteria
Ferrous iron is used as electron acceptor by iron-reducing bacteria such as G. metallireducens, G. sulfurreducens, and Shewanella putrefaciens.Different forms of ferric oxides exist in aaerobic drained as well as in waterlogged soils. Not all of these ferric oxides are equally suitable for reduction by ferric oxide reducer bacteria (Gotoh and Patrick, 1974; Schwertmann and Taylor, 1977). In general, amorphous forms are more efficient for ferric reducer bacteria than crystalline forms (Lovely adn Phillips, 1986). The reduction of ferric oxide may release phosphate and trace elements that are adsorbed to the amorphous ferric oxide and thus enhance availablity of these compounds in the soil (Lovely and Phillips, 1986).
Sulfate reducing bacteria
Bacteria can use acetate as electron donor and sulfate as electron acceptor. This reaction is as follow;
CH3COO- + SO42- + 3 H+ ---> 2CO2 + H2S + 2 H2O
This reaction is carried out by sulfate-reducing bacteria such as Desulfobacterales, Desulfovibrionales, and Syntrophobacterales (Langston and Bebiano 1998). Hydrogen sulfide gas produced via anaerobic respiration cause the rotten egg odor.
Manganese reducing bacteria
Greenhouse Gas Emissions
Green house gases(nitrous oxides, methane, carbon dioxide ) emission from flooded soil(rice paddy,riverine, estuarine, and lacustrine sediments)
References
(1) Kate Scow lecture 5
x Appl Environ Microbiol, June 1998, p. 2181-2186, Vol. 64, No. 6 http://aem.asm.org/cgi/content/full/64/6/2181
Edited by students of Kate Scow
