Yaws
Introduction
Yaws is a bacterial infection that enters through an open lesion and affects
the skin, bones, and joints of its host if left untreated. This infection is caused by a microaerophilic spirochete bacterium, Treponema pallidum pertenue, which flourishes in tropical regions with poor living conditions, such as India, Africa, and South America. Yaws
was nearly eradicated in the mid 1900s, but has recently become a major
problem in many countries, specifically, India. The ecological,
governmental, and social aspects of India along with the nature of the microbe
have contributed to the resurgence of yaws.
Description of Yaws
Higher Order Taxa
Kingdom: Eubacteria
Phylum: Spirochaetes
Class: Spirochaetes
Order: Spirochaetales
Family: Spirochaetaceae
Genus: Treponema
Species: Pallidum
Subspecies: Pertenue
Description of Treponema pallidum subspecies pertenue
Treponema pallidum subspecies pertenue, the causative agent of yaws, cannot be distinguished by means of histopathologic, serologic, immunologic or therapeutic methods from other treponemal bacteria such as Treponema pallidum subspecies pallidum, which causes syphilis and Treponema pallidum subspecies carateum, which causes pinta (16). Treponemes are the specific bacteria that cause yaws, syphilis, and pinta. Each treponeme is only differentiated by mode of transmission, clinical criteria of the disease, and infection of laboratory animals and humans (16). However, a recent study discovered an antigenic difference established by a single amino acid residue at position 40 in the proteins, namely glutamine in TpF1 of subspecies pallidum and arginine in TyF1 of subspecies pertenue (8).
Treponema pallidum is a spirochete bacterium — spiral-shaped with outer and cytoplasmic membranes and a thin peptidoglycan layer. It has periplasmic flagella (or endoflagella), which lie in the periplasmic space and expand from both ends toward the middle of the organism. The flagellar filament has a sheath and core structure, and is composed of at least four major polypeptides (15). T. pallidum contains at least eight major membrane-associated lipoporoteins. The small number of intramembranous protein particles (~70 per mm2) in the outer membrane contributes to its unusual structure compared to other spirochetes and gram-negative bacteria that possess sevenfold the amount of protein. The sparsely distributed and uniformly sized outer membrane particles indicate that there are few different types of protein in the outer membrane. It is hypothesized that the low concentration of surface-exposed protein antigens decrease the reactivity of antibodies and immune cells enabling T. pallidum to avoid immune response, thus causing its pathogenesis (15).
T. pallidum was recently discovered to be a microaerophilic organism with a doubling time greater than 30 hours. Its slow growth and fastidious character in vivo and in vitro suggest that it may have metabolic limitations and growth requirements yet to be identified (15). However, previous studies indicate that it is capable of glucose metabolism and the synthesis of DNA, RNA, and protein. T. pallidum is a parasite and depends on host cells for protection against oxygen radicals because the bacterium need oxygen for metabolism but are highly susceptible to its toxicity (15).
Pathogenesis
Treponema pallidum subspecies pertenue is transmitted intradermally between humans by the transmission of puss through an open lesion. The puss contains treponemes, which enter the host through open abrasions of the skin or mucous membrane (17). Treponemes move through epithelial cells via the tight junctions between cells and invasively attach to fibronectin-coated surfaces on the extracellular matrix of host cells. Attachment to the fibronectin causes increased synthesis of fibroblasts in the cell (1). Antibodies in the circulating blood attach to antigens on the treponemes and ignite an inflammatory immune response that increases the swelling of the lesions (15).
Low concentration of antigen epitopes expressed on the cell surface of T. pertenue is the predicted cause of the pathogenesis of the bacteria because the limited amount of surface antigens decreases the likelihood of a host cell antibody recognizing the antigen (15). One-dimensional Radioimmunoprecipitation (RIP) confirmed that subspecies pertenue had a decreased amount of proteins expressed on the cell surface compared to subspecies pallidum (17).
The antigen that is thought to be immunodominant in T. pertenue is the 47-kDa antigen, which is present in all T. pallidum subspecies. Monoclonal antibodies 11E3 and 13C6 react with this antigen on the bacterial cell surface in the immune response against the bacteria. In comparison study between T. pallidum and T. pertenue, a binding assay and electron microscopy study were done and showed that the antigen was abundant on the cell surface of T. pallidum but had reduced presence on the surface of T. pertenue (12). This study indicated that the reduced presence of the 47-kDa antigen allowed for greater pathogenesis of the cell by reducing its ability to be recognized by host antibodies.
The contrast in the presence of the Immunoglobin M (IgM) and Immunoglobin G (IgG) antibodies in the immune system of neonate and adult guinea pigs indicates a greater risk of infection in children, which validates the prevalence of yaws in children under 15. In a study, adult guinea pigs expressed five times the amount of antibodies after exposure to T. pertenue compared to neonates. Additionally, the presence of antibodies was greatest in adults three to six weeks after infection, while neonates did not reach their peak presence until six to nine weeks after exposure (18).
While the limited presence of antigens aids the pathogenesis of the bacteria, the limited surface proteins also inhibit it. Specifically, the surface proteins P1 and P3, which are fibronectin-binding proteins, are very limited in presence on the cell surface. The absence of these proteins limits the number of host cells that the bacteria can bind to, therefore limiting the exposure of bacteria to the host cells. Fibronectin-binding surface proteins P2, P4, and P5 are not limited in their presence on the cell surface, however, which increases the pathogenesis and contributes to the effectiveness of the bacteria (14).
Many aspects of the pathogenesis of T. pertenue are still unknown and being studied, but the differences in the presence of antibodies, antigens, and other surface proteins provide some insight to the complex pathogenesis of this subspecies of T. pallidum.
Symptoms of Yaws
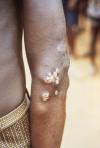
The symptoms of yaws usually appear in 3 stages: primary, secondary, and tertiary. The primary stage involves the entry and initial development of T. pallidum pertenue. The secondary stage is characterized by varying skin lesions and is highly infectious. Secondary lesions may go on to develop into tertiary stage, during which secondary lesions may come and go.
Primary stage:
After an incubation period of T. pallidum pertenue of approximately 3 weeks, the primary lesion (mother yaw) develops after a scratch, bite, or abrasion on exposed skin. The mother yaw develops a honey-brown crust and enlarges horizontally 1-5 cm in diameter. The crust hardens and then sloughs off and reveals a raspberry-like base. This raspberry-like base is filled with treponemes, making these lesions highly infectious. During this stage, infected persons may experience lymphadenopathy (swollen lymph nodes), fever, and joint pain. The mother yaw resolves spontaneously in 2-9 months, leaving an atrophic scar with central hypopigmentation and peripheral hyperpigmentation (10).
Secondary stage:
About 6-16 weeks after the primary stage, an eruption of skin lesions, bone lesions, and constitutional symptoms appears. The cutaneous lesions (daughter yaws) resemble the mother yaw but are smaller (up to 2 cm in diameter) and are usually located next to the mouth and nose. The daughter yaws expand, ulcerate, and excrete a fluid swarming with treponemes, which dries into a crust. These bumps on the skin surface can form thick, hyperkeratotic plaques that may become fissured or eroded. Macular and hyperkeratotic lesions on the palms of the hands and the soles of the feet (similar to lesions found in syphilis patients) may also be present. Infected persons may also experience painful osteopetrosis (bone hardening). Some of these early bone changes can be observed on radiographs. Manifestations during this stage are generally non-scarring and reversible. Patients may develop relapses for up to 5 years after the initial infection. The disease then enters a noninfectious latent period where patients do not exhibit any signs or symptoms (10).
Tertiary stage:
After 5-15 years of latency, a late stage develops and is characterized by destructive skin lesions, bone lesions, and possible neurological and ophthalmologic damage. Enlarging and painless subcutaneous nodules develop and undergo abscess formation, necrosis, and ulceration. Lesions have well-defined edges and an indurate base with granulation tissue and yellowish slough. The ulcers that develop in this stage may become infected and lead to devastating destruction of underlying structures. Furthermore, these ulcers may coalesce and form tracts that heal with keloid formation, which leads to crippling deformities and contractures (10).
Genomics: Yaws versus Syphilis
The genomes of the pathogenic organisms in the genus Treponema are similar and not easily distinguished because of low genetic diversity (3). Genotyping is accomplished by analyzing the apr and tpr gene sequences of all Treponema organisms. Differentiation between syphilis and yaws is important in accurately diagnosing the patient and molecular analysis studies have been conducted in order to better distinguish between the two microbes which cause the diseases (8). Understanding the evolutionary history of the genome is a prerequisite to understanding how to treat both diseases.
The two subspecies of T. pallidum, pertenue and pallidum, share common glutamic acid-rich acidic repeat protein (arp) regions in their genome (11). Since this is a region that is shared between both pathogenic subspecies, molecular distinctions within this region can be made to classify them from one another. Of the four arp repeat motifs found in T. pallidum strains, type II was only identified in subspecies pertenue. Therefore if type II arp motifs were found in a pure T. pallidum sample, subspecies pallidum can not be present. It should be noted that type II motifs have also been identified in T. pallidum endemicum, which causes nonvenereal bejel disease but again, type II has not been identified in syphilis strains. The type II arp amino acid sequence is: R-E-V-E-D-V-P-K-V-V-E-P-A-S-E-R-E-G-G-E (8).
By examining the minor differences in the three subspecies' genomes medical professionals can better diagnose patients who suffer from either yaws or syphilis or both and therefore administer more effective treatments. In developing countries, treatments are administered after merely evaluating visual symptoms, and this less-informed practice can increase the risk of misdiagnosis (8). The arp gene has a potential membrane-spanning domain that expresses a lack of tryptophan residues. These anchors keep proteins attached to the membrane; therefore it is possible that the arp gene may contribute to subspecies pertenue's lack of surface proteins. Because the arp is most effectively studied as an identifier region, there is little evidence to positively support any arp-functionality theories (11).
The tpr gene family comprise 2% of the T. pallidum genome and probably evolved through gene duplication and gene conversion throughout its evolutionary history (5). The function of tpr is not entirely known but it may be to create antigenic diversity for the pathogen (5). There are three subfamilies within this gene and they can be analyzed to study the evolution of all subspecies within the genus Treponema. It is evident that subspecies pertenue is more closely related to subspecies pallidum than to subsecies endemicum. The close genetic relationship is due to various recombination events between the two subspecies (5). Because there is little genetic difference between their sequences it is doubtful that the evolution of yaws and syphilis were separated by a dramatic time frame, as evidenced by their phylogenetic trees.
Prevention
There are various antibiotics such as tetracycline that can be administered for treating patients infected with yaws. However, the most prevalent drug used in treating any stage of yaws is penicillin G benzathine because its side effects are minimal compared to its counterparts (10). For instance, tetracycline can increase hypoprothrombinemic resulting in delayed clotting of the blood or may cause spontaneous bleeding. Penicillin G benzathine is considered as bactericidal because it affects cell wall biosynthesis during active growth. A single injection can kill the treponemes in a few minutes, while lesions are considered healed within almost a day (10). According to a study in the late 1900s, other treponemal diseases such as endemic syphilis (Treponema pallidum pallidum) are closely related to yaws in which there are no distinct differences in their antigenic and immunogenic properties. Also, bone lesions and joint lesions, which are frequent in yaws and endemic syphilis, show no distinct differences in appearance (6). Similarly, endemic syphilis can be treated with penicillin G benzathine.
Yaws in India
Ecology of Yaws in India
Yaws is particularly prevalent in the districts of Bengal, Travancore, Assam, Burma, and Ceylon (9). Sociological and geographical factors have caused a potential problem in this region of the world that has expanded to be sources of other predicaments economically and politically. Mass serological testing has revealed a high number of cases of yaws around the world. India resulted in 25-30% positive tests of several different population groups tested for serological reactors (7). This has led to the search for solutions and medical responses to eradicate the spread of yaws.
Environmental factors revolve mainly around the moisture content and temperature in tropical and sub-tropical areas (7). High humidity specifically targets high moisture in the skin and an increase in skin infections from diseases and bacteria. High temperature has been observed especially in the areas of Burma and Assam, which falls within the isotherm region of 80°F (9). However, during the winter season, when temperatures have fallen to approximately 65°F on average, yaws has a higher tendency to resemble syphilis, where it may change its form and be nurtured (9). During the winter months, it is rare to see the painful lesions and the characteristic yellow-encrusted yaws on the skin that is usually occurs in warm, moist regions. These attributes are frequently mistaken as syphilitic. Yaws has also been found to be more prevalent in the hills of Assam than on the plains, where the altitude nears the 80°F isotherm region. The hills have been recorded at heights between 1,000 and 5,000 feet above sea level (9).
There is also an increase in the number of cases where rainfall is heavy in India, particularly during the rainy seasons. An increase in new cases and an increase in relapse cases are caused by the heavy rainfall of wet seasons, based on altitude and its soil characteristics. For instance, Burma has a close proximity to the sea and it approaches the 80°F isotherm region. The soil is damp, and because of limited evaporation, water is unable to drain away the limestone that geologically forms. When limestone is present, there is very little vegetation, which becomes another contribution to high incidence of yaws. On the contrary, ample vegetation means scant formation of limestone (9).
In regards to the sociological aspect of India, 10.2% of the 30,000 cases of yaws in India assessed on a population of 1.25 million were Indian. Annually, about 5,000 cases are usually reported (9). Males are more dominant in catching the disease than females. Most cases were found in boys of ages between 5 and 14 years (9).
Economically, a lack of proper sanitation is a large issue in populations where there is a lower standard of living. Overcrowding in huts (2) becomes very common in large populations in India and poor personal hygiene is assumed to be one of the main causes of yaws. The prevalence of yaws can decline in these regions if clothes and shoes were worn more often, which would help maintain personal hygiene and sanitation (9). This serves as a protection barrier from being exposed directly to bacteria-rich objects, including exposure to open sores on infected people.
The availability of food is also scarce in India, which contributes to the low economic status and poor diet of the people. The diets are generally unbalanced: fresh fruit, vegetables, milk, and eggs are not readily available year-round. The water supply is also tainted by overuse, further contributing to poor hygiene (2). Malnutrition and poor sanitation play a major part in the proliferation of Yaws in India.
Health Initiatives: Past and Present
Mass treatment of yaws in India and other tropical countries sponsored by the World Health Organization observed a dramatic fall in the prevalence in the mid to late 1900s (6). Unfortunately, yaws rebounded due to lack of public health surveillance and inadequate treatment facilities in rural areas, while health institutions all over the world poured their attention into other deadly diseases occurring at the same time (6). From 1997 to 1998, the government of Gujarat in India implemented NSPCD (National Surveillance Programme for Communicable Disease), which aims to promote community participation, strengthen communication between district level and sub-district level, detect early warning signs and provide rapid response for treatment, and improve laboratory based surveillance for effective diagnoses and treatment services (4). Afterwards, more districts followed suit and specifically YEP (Yaws Eradication Programme) was launched as a pilot study in Koraput district, which later on extended to 49 other districts (19). The goal of YEP is to eliminate the yaws disease in India and ultimately eradicate the disease in the foreseeable future. The strategies are active case detection and treatment by using antibiotics (19). However, in other countries such as Papua New Guinea, cases of re-infection are occurring, suggesting increased tolerance of some Treponema pallidum pertenue strains to the common penicillin treatment, but no such case has been found in India (10).
Conclusion
Yaws is a preventable disease that can be readily eradicated in India and throughout the world. The initial eradication efforts in the mid 1900s focused on mass treatment of the infected communities in impoverished areas with penicillin. This method was effective then and continues to be today. After the initial efforts, yaws was overlooked as other major endemics and epidemics came forth and became the primary focus of most health organizations. This error can be attributed as one of the primary reasons for the comeback of yaws to many tropical regions. With yaws on the rise, treatment with penicillin is the primary method for treatment, prevention, and hopefully eradication worldwide. The effectiveness of this treatment method in the past on a mass scale makes penicillin treatments the best option for future treatment. Prevention and eradication, however, depend on health organizations and governments in India and all impoverished countries to help the people. Proper clothing and food sources in these areas are the best way to stop the disease from spreading, which is the only way for complete eradication.
References
1 – Cameron, C., Elizabeth L. Brown, Janelle M. Y. Kuroiwa, Lynn M. Schnapp, and Nathan L. Brouwer. “Treponema pallidum Fibronectin-Binding Proteins”. Journal of Bacteriology. 2004. Vol. 186. P. 7019-7022. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=15466055
2 - Cutler, John C., Johs. Kvittingen, Evelyn Rose, James C. McCullough, R.B. Tampi, Sukumar Sen, Chandra Parmar, and Girdharil Lal. "Mass treatment of syphilis in an Indian province: Report of the World Health Organization Venereal Disease Demonstration Team in Ghund area of the Himachal Pradesh, India." Bull World Health Organization. 1952. Vol. 5. P. 377-439. http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=2554066&pageindex=9#page
3 - Florindo, C., Reigado, V., Gomes, J., Azevedo, J., Santo, I., Borrego, M. “Molecular Typing of Treponema pallidum Clinical Strains from Lisbon, Portugal”. Journal of Clinical Microbiology. 2008. P. 3802-3803.
4 – “Government of Gujarat”. Health & Family Welfare Department. 2005. http://gujhealth.gov.in/health_programmes/epidemic/nspcd.htm#related
5 - Gray, R., Mulligan, C., Molini, B., Sun, E., Giacani, L., Godornes, C., Kitchen, A., Lukehart, S., Centurion-Lara, A. “Molecular Evolution of the tprC, D, I, K, G, and J Genes in the Pathogenic Genus Treponema”. Mol. Biol. Evol. 2006. Vol. 23(11). P. 2220–2233.
6 - Grin, E. "Endemic Syphilis and Yaws". World Health Organization. 1956. Vol. 15. P. 960-969. http://whqlibdoc.who.int/bulletin/1956/Vol15/Vol15-No6/bulletin_1956_15(6)_959-973.pdf
7 - Guthe, T., F.W. Reynolds, P. Krag, and R.R. Willcox. "Mass Treatment of Treponemal Diseases, With Particular Reference to Syphilis and Yaws." British Medical Journal. 1953. Vol. 4810(1). P. 594-598. http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=2015599&pageindex=2#page
8 - Harper, K., Liu, H., Ocampo, P. "The Sequence of the acidic repeat protein (arp) gene differentiates venereal from nonvenereal Treponema pallidum subspecies, and the gene has evolved under strong positive selection in the subspecies that causes syphilis." FEMS Immunol Med Microbiology. 2008. Vol. 53. P. 322-332. http://www3.interscience.wiley.com/cgi-bin/fulltext/119881342/PDFSTART
9 - Hill, Kenneth R. "Non-specific factors in the epidemiology of Yaws." Bull World Health Organization. 1953. P. 17-27. http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=2554286&pageindex=1#page
10 - Levine, Caroline. "Yaws." 1994-2009. http://emedicine.medscape.com/article/1053612-overview
11 - Liu, H., Rodes, B., George, R., Steiner, B. “Molecular characterization and analysis of a gene encoding the acidic repeat protein (Arp) of Treponema pallidum”. Journal of Medical Microbiology. Vol. 56. 2007. P. 715-721.
12 – Marchitto, Kevin S., Susan A. Jones, Ronald F. Schell, Priscilla L. Holmans, and Michael V. Norgard. “Monoclonal Antibody Analysis of Specific Antigenic Similarities Among Pathogenic Treponema pallidum Subspecies”. Infection and Immunity. 1984. Vol. 45. P. 660-666. http://iai.asm.org/cgi/reprint/45/3/660
13 – Noordhoek, Gerda T., Alan Cockayne, Leo M. Schouls, Rob H. Meloen,
E. Stoelz, and Jan D. A. Van Embden. “A New Attempt to Distinguish Serologically the Subspecies of Treponema pallidum Causing Syphilis and Yaws.” Journal of Clinical Microbiology. 1990. Vol. 28. P. 1600-1607. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=267996&blobtype=pdf&tool=pmcentrez
14 – Noordhoek, Gerda T., Peter W. M. Hermansa, Annet N. Paula, Leo M. Schoulsa, Jaap J. Van Der Sluisb and Jan D. A. Van Embdena. “Treponema pallidum subspecies pallidum (Nichols) and Treponema pallidum subspecies pertenue (CDC 2575) differ in at least one nucleotide: comparison of two homologous antigens”. Microbial Pathogenesis. 1989. Vol. 6. P. 29-42. http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6WN6-4C5H4HR-2J-1&_cdi=6954&_user=4429&_orig=search&_coverDate=01%2F31%2F1989&_sk=999939998&view=c&wchp=dGLzVzz-zSkzV&md5=90d7514c0834ee266bbeec2fb19dd21d&ie=/sdarticle.pdf
15 – Norris, S. J. “Polypeptides of Treponema pallidum: Progress toward Understanding Their Structural, Functional, and Immunologic Roles”. Microbiological Reviews. 1993. Vol. 57. P. 750-779. http://mmbr.asm.org/cgi/reprint/57/3/750
16 - Smith, J., and Israel, C. “A Neuro-Opthalmologic Study of Late Yaws and Pinta”. Department of Opthalmology. 1970. Vol. 68. P. 292. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1310380
17 - Thornburg, R., and Baseman, J. “Comparison of Major Protein Antigens and Protein Profiles of Treponema pallidum and Treponema pertenue”. Infection and Immunity. 1983. Vol. 42. P. 623-627. http://iai.asm.org/cgi/reprint/42/2/623
18 – Wicher, Konrad, Victoria Wicher, Frank Abbruscato, and Robert E. Baughn. “Treponema pallidum subsp. pertenue Displays Pathogenic Properties Different from Those of T. pallidum subsp. pallidum”. Infection and Immunity. 2000. Vol. 68. P. 3219-3225. http://iai.asm.org/cgi/reprint/68/6/3219?ck=nck
19 - World Health Organization. "Yaws elimination in India: a step towards eradication." Communicable Diseases. Country Office for India. 2007. http://www.whoindia.org/en/Section210/Section424.htm
Edited by Raven Bates, Maelen Ignacio, Yin-chen Lee, Beverly Pitogo, Joseph Sullivan, and Adrian Tubongbanua, students of Rachel Larsen
