Hepacivirus
Baltimore Classification
Higher order taxa
Viruses; ssRNA positive-strand viruses, no DNA stage; Flaviviridae; Hepacivirus
Species
Hepatitis C virus
Description and Significance
Hepatitis C virus was first identified with certainty by molecular cloning of the virus genome in 1989 and was originally the one source of non-A non-B hepatitis. Investigation of the virus has been greatly hampered because it cannot be cultured in vitro.
The genome of the virus resembled that of a flavivirus. The positive stranded RNA genome is around 10000 bases long. The structural genes are at the 5- end while the non-structural genes are at the 3' end. The morphological structure of the virus remains unknown, and the virus has not been visualized convinvingly in the electron microscope yet. (source: Virology-Online)
Genome Structure
The genome of hepatitis C virus is not segmented and contains a single molecule of linear positive-sense, single stranded RNA. The complete genome is fully sequenced and 9600 nucleotides long. The sequence has the accession number [M62321]; [M58406]; [M58407]; [D90208]; [M58335]. The 5'-end of the genome does not have a cap while the3'-terminus has a long non-coding region; conserved nucleotide sequences and a sequence variable region. The sequence is about 100 nucleotides in length and has higly conserved regions. The 3'-terminus has variable regions and has 50 nucleotides in length. It also has a polypyrimidine region which varies in length but about 100 nucleotides long in average. The 3'-terminus usually does not have a poly (A) tract but some isolates do. The genome has an intergenic poly (A) region. (source: ICTV dB Descriptions)
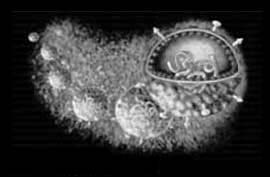
Virion Structure of a Hepatitis C virus
The virions of hepatitis C virus consist of an envelope and a nucleocapsid. A detergent sensitive lipoprotein envelops the virus capsid. Virions are spherical and are estimated to be about 50 nm in diametyer. The capsid is round and exhibits polyhedral symmetry. The core is isometric and has a diameter of about 30 nm. (source: ICTV dB Descriptions)
Reproductive Cycle of a Hepatitis C virus in a Host Cell
Although very little is known about the replication cycle of hepatitis C virus because there is no in vitro cell culture system permisive for virus replication, progress is being made in this regard.
The replication strategy of hepatitis C virus is similar to that of other positive-strand RNA viruses. The virus enters the cell and is uncoated in the cytoplasm. The viral genome is transcribed to form a complementary negative-sense RNA molecule. This molecule serves as a template for the synthesis of progeny positive-strand RNA molecules. A host-cell signalase as well as virus-specific non-structural proteins NS-2 and NS-3 then clear the newly translated polyprotein. The enzyme capable of performing both steps of RNA synthesis is the virally encoded RNA-dependent RNA polymerase NS5b.23. The NS-3 of hepatitis C virus also has helicase activity. The hepatitis C virus replicates by a negative-strand RNA intermediate and has no reverse transcriptase activity. (source: World Health Organization: Epidemic and Pandemic Alert and Response (EPR))
Viral Ecology & Pathology
The incidence of post-transfusion hepatitis has declined since HBsAg screening was introduced in America. A significant number of cases, however, still remained with non-A non-B causing at least 95% of post-transfusion hepatitis. There are thought to be 100 million carriers of hepatitis C carriers in the world. Japan is a hotspot for hepatitis C infection and it is thought to ve responsible for the majority of cases of hepatocellular carcinoma. In the US alone, there are 175,000 new cases of hepatitis C each year.
The prevalence of antibody to HCV (anti-HCV) in serum in most developed countries ranges between 1% and 2%, and infection with HCV occurs worldwide. Parts of eastern Europe and Africa have been observed to have significantly higher rates of infection while Egypt seems to have one of the highest prevalence rates of all, approaching 15% of the general population. In the USA for example, approximately 0.5% of volunteer blood donors are anti-HCV positive, while among a random sample of the general population 1.8% test positive for anti-HCV. The latter data has allowed the Centers for Disease Control to estimate that nearly 4 million people are infected with HCV in the USA.
Each clinical form of viral hepatitis B virus can also occur with hepatitis C virus. Acute disease may lead to recovery, fulminant hepatitis, relapsing hepatitis with intervening periods of normal liver function, inapparent chronic infection, chronic active hepatitis and cirrhosis have been documented. The incubation period for hepatitis C virus lies between that of hepatitis A virus and hepatitis B virus. Hepatitis C virus infection is particularly associated with high ALT levels and it was suggested that ALT levels could be used as surrogate marker for the screening of NANBH before the availability of a test for anti-hepatitis C virus.
Of all patients infected with hepatitis C virus, 50-75% of the patients are thought to be suffering chronic infection. In a cohort study where patients with hepatitis C virus chronic diseases were followed up for 10 years, it was observed that 20% had cirrhosis, 30% made improvements, 45% remained stable and 25% have shown progression, including the development of cirrhosis.
Several recent studies have established a strong connection between hepatitis C virus and hepatocellular carcinoma (HCC). In the absence of hepatitis B markers, anti-hepatitis C virus antibodies were detected in 44.5% of HCC patients in Spain, 43% in Japan, 16%in Italy, and 7% in South Africa. Since hepatitis B carriers often have chronic hepatitis C infection, it is possible that the two viruses may act together to cause HCC and epidemiological data linking hepatitits B virus to HCC may require reassessment in terms of the magnitude of the risk involved with the availability of testing for chronic hepatitis C virus infection. (source: Virology-Online)
References
Adrian M Di Bisceglie; "Hepatitis C"; The Lancet; Vol 351, January 31, 1998
World Health Organization: Epidemic and Pandemic Alert and Response (EPR)