Gonorrhoea (Neisseria gonorrhoeae) in the United States
Introduction
With over 600,000 new infections reported each year, Neisseria gonorrhoeae (N. gonorrhoeae) has become the second most common, sexually transmitted infection in the United States. With its incidence only second to Chlamydia trachomatisa [6], the most disturbing fact of N. gonorrhoeae lies in the reality that it shows no discrimination among the sexually active - anyone and everyone is susceptible. The group most at-risk are sexually active women under the age of twenty-five and African American men [4]. Other risk factors include previous gonorrheal infection, diagnosis of other sexually transmitted diseases, new or multiple partners, inconsistent-to-no condom use, commercial sex work, as well as drug use [6]. Notable symptoms in males may include swollen and painfully sensitive testes, a white, yellow, or green penal discharge, a burning sensation while urinating, and/or the formation of unusual sores/a rash. In females, symptoms may include a painful or burning sensation while urinating, vaginal bleeding between periods, and/or increasing vaginal discharge. Due to the fact that the majority of those infected are asymptomatic, especially among women, if left untreated, the infection can result in the sterility in either sexes and in extreme cases, lead to their fatality [6]. Thus, the only factor keeping the infection from developing into an epidemic rests upon people routinely getting tested. Fortunately, because N. gonorrhoeae is a bacterium, curable treatment is available. However, this relief is currently being threatened.
History of the Name Neisseria Gonorrhoeae (N. Gonorrhoeae)
Aelius Galenus, a Roman physician and philosopher, first observed the bacterium in the second century and loosely named it the "flow of seed" [18]. The term resurfaced in the sixth century when Aetius Amidenus, a Byzantine physician and medical writer, observed the bacterium in pelvic abscesses. Infectiously spreading to France, the term gained popularity as it became symptomatically renown as "La chaude pisse", which literally translates to "hot piss". Finally, in 1879, Albert Ludwig Sigesmund Neisser, a German physician and bacteriologist, was the first person to describe the bacterium as the causative agent of gonorrhea and officially named the bacterium Neisseria gonorrhoeae [14].
Description of the Microbe
Neisseria gonorrhoeae (N. gonorrhoeae) is a gram-negative diplococcus ranging from 0.6 to 1.0 µm in diameter and is usually identified in pairs with adjacent flattened sides. Its outer membrane is composed of proteins, phospholipids, and lipopolysaccharides (LPS). The N. gonorrhoeae lipopolysaccharide can be distinguished from other types of LPS by "its highly-branched basal oligosaccharide structure and the absence of repeating O-antigen subunits" [18]. Thus, it is referred to as lipooligosaccharide (LOS). During its growth, N. gonorrhoeae releases its outer membrane fragments called "blebs" that contain LOS, an endotoxin that plays a primary means in the bacterium's pathogenesis [18]. LOS is involved in infection by attaching to host cells, evading the host's immune system via antigenic variation, damaging tissue, and manifesting bactericidal antibodies [8].
The antigenic variation of N. gonorrhoeae manifests via rearrangements of its chromosomal DNA by the altering of its expression of any one of the several silent pilin genes it can possibly encode [19]. By doing so, phase variation occurs when the rearrangement involves a defective pilin gene, thus avoiding their host (i.e., humans) immune system [13, 19]. Its fimbriae and antigenic type IV pilus enables the bacterium to move, while the opacity (Opa) proteins provide adherence to host cells. Together, they form tight junctions and are constituted of repeated peptide subunits characterized by both antigenic and phase variations [18]. The bacterium, like N. meningitidis, is also "naturally competent for DNA uptake" after attachment [10].
Through such means, N. gonorrhoeae infects the mucous surfaces that are lined with columnar epithelium cells by the attachment of the bacterium via pili (fimbriae) and the production of lipopolysaccharide endotoxin [13, 18]. Due to the fact that the lipopolysaccharide is highly toxic and contains virulence factors in the form of its antiphagocytic capsule which produces IgA proteases, they contribute to the infection [18]. The bacterium is typically observed inside polymorphonuclear leukocytes (i.e., neutrophils) that become a part of the gonorrhea pustular exudate.
Overall, N. gonorrhoeae is a fragile bacterium that is sensitive to environmental conditions including temperature changes, drying, ultraviolet light, and the such. It is a "fastidious" bacterium that "requires hemoglobin or blood, several amino acids, and vitamins" for nourishment. In the laboratory, cultures must be grown at optimal temperature of 35-36 degrees in an atmosphere of 3-10% carbon dioxide [13].
Transmission
Gonorrhea, which is caused by the bacterium N. gonorrhoeae, is harbored in warm, moist areas. It is sexually transmitted through the contact of an infected tissue surface on the vaginal, penile, anal, and/or oral cavity and another susceptible mucosa epithelium surface [17,11]. In addition to being sexually transmitted, gonorrhea infection may also be trasnmitted vertically - from mother to child during delivery [11].
Symptoms
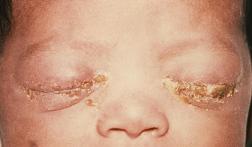
The overall symptoms of gonorrhea vary substantially between the sexes and can be fatal if left untreated. If sexually active, it is safest to routinely get tested and critical to seek medical attention if any of the following symptoms below are observed. Among males, signs of symptoms can appear anywhere from 2 to 30 days after sexual intercourse, or not at all. Such symptoms may include the following: a white, yellow, or green penal discharge, a burning sensation while urinating, and/or the formation of unusual sores/a rash. Occasionally, males can also get swollen and/or feel pain in their testicular regions. If left untreated, males can develop epididymitis, prostatitis, urethritis, become sterile, and potentially die. Among females, it is common for symptoms to appear meek or asymptomatic. If symptoms are observed, they may include the following: a painful or burning sensation while urinating, vaginal bleeding between periods, and increasing vaginal discharge. If left untreated, females may than develop pelvic inflammatory disease, potentially become sterile, and die [6].
Why is the Microbe a Problem in the United States?
A myriad of sources are responsible for the perpetuation of gonorrhea in the United States: ??????????????????????????????????????? - no commitment to regular check-up's - denial - "just world" belief - sexual liberalism (70's) - sexual performance drugs - teenage culture - asymptomatic therefore easily and unknowingly transmitted and reinfected - population density in metropolitan areas increase social interaction, networks, and therefore relationships - increase in antibiotic resistance - no open communication/dialogue among sex partners - safe sex practices being neglected
Sexually active women under the age of twenty five and African American men have the highest reported rates of N. gonorrhoeae [3]
Due to all of the above, according to the New York Times article written by Lawrence K. Altman titled, Sex Diseases Still Rising: Chlamydia Is Leader, gonorrhea infections have been on the rise.
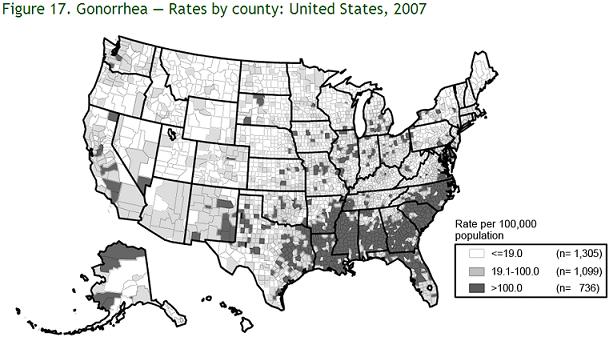
In comparison 18 to 1 ratio of African Americans to Whites. “African Americans account for 69 percent of all gonorrhea in this country” [1]. Also it is important to note according to the newsletter written in DRUG WEEK on an MMWR Report on Gonorrhea and Chlamydia Incidence among MSM, that gonorrhea or Chlamydia infections can be significant co-factors in the transmission of HIV [2]. This becomes another issue in America as it is the harbor of many homosexuals living in big cities such as San Francisco, Los Angeles, and New York, that such infections can easily be transmitted to many individuals in a very short amount of time. Often drug use is the highest in these areas, which can further cause a much higher incidence rate due to sharing of potentially infected needles.
What is Being Done to Combat the Spread of N. Gonorrhoeae?
As the second most commonly reported sexually transmitted disease, a multitude of prevention and treatment strategies have been developed and enforced to control the spread of gonorrhea. Due to the fact that the prevalence of N. gonorrhoeae infection varies among communities and populations [6], the basic sociological measures for control include preventative solutions such as: (1) increasing the education and awareness of the N. gonorrhoeae microbe and its resulting gonorrhea infection, (2) openly supporting abstinence, monogamous relationships, and condom use, (3) encouraging those at-risk be tested, (4) stressing the urgency in seeking treatment if diagnosed and refraining from sexual intercourse until remission, and (5) referring the sex partner of the diagnosed be tested [4, 9]. At the clinical front, physicians are doing their part to control N. gonorrhoeae transmission by testing all at-risk groups – the highest at-risk groups being sexually active women under the age of twenty-five and African American men [4]. Other risk factors include previous gonorrheal infection, diagnosis of other sexually transmitted diseases, new or multiple partners, inconsistent-to-no condom use, commercial sex work, as well as drug use. The effective diagnostic tests at which physicians are currently utilizing to test for N. gonorrhoeae include: Gram stain (reserved for symptomatic patients only), culture, nucleic acid hybridization tests, and nucleic acid amplification tests (NAAT). Each requires the testing of cervical, vaginal, male urethral, and/or urine specimens. Fortunately, if a positive test for N. gonorrhoeae results, due to the fact that the microbe is a bacterium, it can easily be treated and cured with antibiotics such as ceftriaxone, cefixime, ciprofloxacin, ofloxacin, or levofloxacin. However, with N. gonorrhoeae’s rising co-infection with uncomplicated C. trachomatis and health officials’ routine approach to co-treat both bacterial infections, an antimicrobial-resistant N. gonorrhoeae strain known as quinolone-resistant N. gonorrhoeae (QRNG) has developed. As a result, common antidotes containing fluoroquinolones, such as ciprofloxacin, are inadvisable, especially in the United States. Two states of particular concern are California and Hawaii as they both contain the highest prevalence of QRNG as seen in the Centers for Disease Control and Prevention’s Gonococcal Isolate Surveillance Project 2004 study of 6,322 isolates showing 6.8% resistant to ciprofloxacin. Excluding the isolates gathered from California and Hawaii, only 3.6% were QRNG [6]. Overall, this study’s conclusion is critical as it highlights the competitive race between aggressively adapting antibiotic-resistant microbes and the scientific discovery of new antibiotics.
What Else Can be Done to Address this Rising Problem?
As antibiotic-resistant strands of N. gonorrhoeae continue to spread throughout the United States, certain antibiotics are increasingly becoming ineffective in treating gonorrhea. Since 1993, fluoroquinones have been used frequently to treat gonorrhea in the United States because it was the overall drug treatment of choice; fluoroquinones were effective as an easy oral method of treatment that required only a single dose. In 2006, however, the Centers for Disease Control and Prevention (CDC) announced that it no longer recommends fluoroquinones for treatment of gonorrhea because of the bacterium's increasing resistance throughout the United States. Similarly, other antibiotics such as penicillin, tetracycline, and macrolides have not been recommended in the United States because susceptibility to such drugs has been inadequate [6]. Currently, drugs within the cephalosporin class are being used to treat gonorrhea in the United States, but growing resistance and adaptation to antibiotics places heavy emphasis on the development of novel pharmaceuticals for future treatment of gonorrhea. Current research seeks to uncover the genetic and molecular specifics of N. gonorrhoeae, such that particular drugs may be targeted to disrupt it. For example, antigenic variation has been found in N. gonorrhoeae, thus making it hard to develop a vaccine against it [14]. Thus, more focused, pharmaceutical studies geared toward uncovering the molecular basis by which antigenic variation occurs would prove itself useful for combating antibiotic-resistant strands of N. gonorrhoeae [16].
Along with the development of novel drugs for treatment, the development of novel techniques to diagnose individuals with gonorrhea would also be effective toward curbing the spread of the infection. One such recently developed technique that has gained the respect of being the most sensitive and specific test to date for N. gonorrhoeae detection is the nucleic acid amplification test (NAAT)— of which polymerase chain reaction (PCR) falls into categorically. Despite the effectiveness of NAAT, the Food and Drug Administration (FDA) has not cleared for its use on rectal and pharyngeal tissues [4] due to the fact that native microbes that inhabit those areas compromise test results. Future developments may empirically test NAAT to ascertain its usefulness for diagnosing such areas, thereby lifting the FDA restriction.
Another mode for preventing further spread of gonorrhea is through the development of more hubs for disease control (e.g., clinics), particularly in metropolitan areas where infection is most prevalent due to high population density and increased social and sexual interaction. By constructing more clinics, a more accurate representation of the epidemiology of N. gonorrhoeae may be ascertained. The means by which the United States currently monitors and controls N. gonorrhoeae epidemiology is through the Gonococcal Isolate Surveillance Project (GISP). GISP monitors N. gonorrhoeae prevalence and its susceptibility to antibiotics through sexually-transmitted disease (STD) clinics, regional laboratories, and the CDC itself. It was through GISP that resistance to a fluoroquinone was first identified in 1991. In GISP protocol, a representative subset of the cultures positive for N. gonorrhoeae are tested for antibiotic resistance. As a result, specific antibiotics are prescribed based on demographic and regional data such that treatment remains effective. Currently, approximately 28 cities with STD clinics are part of GISP. With more hubs of disease control, the accessibility of preventative education, materials, and treatment would become available to better tailor to regional needs [3].
References
1. Altman, Lawrence K. "Sex Diseases Still Rising; Chlamydia Is Leader." The New York Times 14 Nov. 2007: 21-21. Print.
2. Barry, P. M. "Drug Week." GONORRHEA; Research from P.M. Barry and co-authors yields new data on gonorrhea (20 Mar. 2009): 616. Print.
7. Crosby, R.A., R.J. DiClement, S.L. Rosenthal, and L.F. Salazar. Prevention and Control of Sexually Transmitted Infections Among Adolescents: The Importance of a Socio-Ecological Perspective. ScienceDirect 2005; 119.9:825-36. Print.
10. "Genome Project Result." NCBI HomePage. Web. 25 Aug. 2009.
11. "Gonorrhea." National Institute of Allergy and Infectious Diseases. Web. 26 Aug. 2009.
13. "Gonorrhea." Welcome to the UW-Madison Dept. of Bacteriology. Web. 26 Aug. 2009.
14. Hedges SR, Mayo MS, Mestecky J, Hook EW III & Russell MW (1999) Limited Local and Systemic Antibody Responses to Neisseria Gonorrhoeae During Uncomplicated Genital Infections. Infect Immun 67: 3937–3946. Print.
15. J. O'Dowd, Michael. The History of Medications for Women. New York City: The Parthenon Publishing Group Inc., 2001. Print.
16. Pilin Gene Variation in Neisseria Gonorrhoeae: Reassessing the Old Paradigms. FEMS Microbiology Reviews [0168-6445] Hill, Stuart (2009) volume: 33 issue: 3 page: 521 -30. Print.
17. "STD Facts - Gonorrhea." Centers for Disease Control and Prevention. Web. 26 Aug. 2009.
19. "WHO | Sexually Transmitted Diseases." Web. 25 Aug. 2009.
Edited by: Joelle Abed Elahad, Mykel Anderson, Tim Chiang, Danny Huynh, Fisal Mohsini, and Cuong Nguyen, students of Rachel Larsen, Ph.D.