Venezuelan equine encephalitis virus
Venezuelan equine encephalitis virus (VEEV)
A Viral Biorealm page on the family Venezuelan equine encephalitis virus
Baltimore Classification
Group IV: (+) sense single-stranded RNA viruses
Higher Order Categories
Family: Togaviridae
Genus: Alphavirus
Complex: New World (occurring only in the Americas)
Description and Significance
Venezuelan equine encephalitis virus (VEEV) is an important animal and human pathogen that was weaponized by the U.S. military in the 1960’s. [3]. Although it was weaponized it is still only considered a category B bioterrorism threat by the Centers for Disease Control and Prevention because it causes only moderate morbidity and low mortality rates. It is categorized as biosafety level 3 (BSL 3) agent (Bio safety level 3) which requires researchers to work in a negative pressure laboratory with extensive safety protocols. [4]
VEEV is part of a group of alphaviruses that cause arthritic and encephalitic disease in birds, horses, rodents and humans. This group includes O'nyong'nyong virus, Chikungunya virus, Western equine encephalitis virus, and Eastern equine encephalitis virus. Alphaviruses are not just importance human and animal pathogens but have also been used as a model system in the study of enveloped virus structure and as viral vectors in gene therapy [add the gene therapy thing].[1] (sounds weird)
Genome Structure
VEEV has an 11.5 kb (+) sense ssRNA genome. The genome contains two reading frames that encode for a polyprotein containing capsid & envelope proteins (5 proteins) and a polyprotein involved genome replication (4 proteins including viral RNA-dependent RNA polymerase). (MORE HERE!)more about the genome and the stuff it codes for. something about a poly-A tail-like other (+) sense RNA viruses
The 5’ ORF codes the non-structural polyprotein which includes three helicase domains, a 3C-protease domain, and 8 RNA polymerase domains (including an RNA-dependent RNA polymerase). The 3’ ORF codes for the structural polyprotein which contains two picornavirus-like capsid protein domains and other capsid proteins. [6]
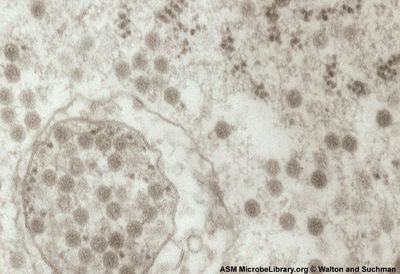
Virion Structure of Venezuelan equine encephalitis virus
Venezuelan equine encephalitis virus is an enveloped isohedral virion. (sounds bad) consists of a non-enveloped, icosohedral capsid. The capsid is constructed from 3 structural proteins [5]. The capsid appears round and is approximately 30 nm in width [8].
Reproductive Cycle of Venezuelan equine encephalitis virus in a Host Cell
Little is specifically known about the KBV reproductive cycle. Some insight into the process can be gained by examining related viruses and examining the patterns of viral RNA and protein expression in infected bees.
The infective ability and life cycle of Rhopalosiphum padi virus (RhPV), also of the Cripavirus genus, has been studied to some extent. In cell culture, viral protein is localized to the cytoplasm, as visualized by immunogold labeling. This study also observed clusters of single membrane vesicles associated with virions in the early stages of infection. Crytalline arrays of virus particles and dense amorphous cytoplasmic structures were also observed.[3] Similar observations have been made for related viruses so it is reasonable to think that KBV infection may follow a similar scheme.
Another clue can be taken from the apparent ability of KBV to maintain a latent infection. Since KBV does not have a DNA intermediate, it cannot enter true lysogeny and splice into the host genome but it can remain in the cell without any observable pathology. Molecular evidence for latent infection was found when ELISA (immuno assay)results for viral-capsid proteins and RT-PCR amplification of viral RNA where compared as diagnostic techniques. In a majority of cases, viral RNA was present with little or no viral-capsid proteins.[9] This suggests that the viral RNA can persist in infected cells with limited replication and formation of new virus particles.
Viral Ecology & Pathology
animal hosts
skeeters are vectors-what species specifically
what it does-mostly flu-like illness, no biggie BUT can cause encephalitis and death
KBV is known to infect many species of bees across a large geographic distribution. Cases of A. melifera infection have been reported worldwide, although cases involved in CCD have only been reported in the US. KBV is also known to infect A. cerana (Asiatic honey bee) in parts of Southeast Asia and India, where the virus is believed to have originated. KBV has also been reported in populations of Bombus spp. (bumble bees) in New Zealand and Vespula germanica (European wasp) in Australia. [6] Some evidence shows that mites such as Varroa destructor not only act as vectors for viral sread, but also as hosts in which viral replication occurs [9].
KBV is spread between bees in a colony and between colonies through many methods of transmission. KBV can be introduced to hives by mites such as Varroa destructor [4] and can also be spread between worker bees and from the queen bee to her offspring. Viral RNA has been amplified from queens and their eggs suggesting that the virus can be transferred via a transovarial route. Viral RNA has also been amplified from food sources such as honey, brood food, and royal jelly. Since these food sources are partially composed of secretions from worker bees, this is a likely method of bee-to-bee transmission. [9]
As mentioned previously, KBV infection of shows no visually detectable signs of pathology [9]. KBV is commonly found in bees co-infected with other related viruses.
References
[1] Jose, J., J.E. Snyder, R.J. Kuhn. “A structural and functional perspective of alphavirus replication and assembly.” Future Microbiology 4 (2009): 837-856
[2] Berenyi, O., T. Bakonyi, I. Derakhshifar, H. Koglberger, N. Nowotny. “Occurrence of Six Honeybee Viruses in Diseased Australian Apiaries.” Applied and Environmental Microbiology 72.4 (2006): 2412-2420.
[3] Boyapalle, S., N. Pal, W.A. Miller, B.C. Bonning. “A glassy-winged sharpshooter cell line supports replication of Rhopalosiphum padi virus (Dicistroviridae). Journal of Invertebrate Pathology 94 (2007): 130-139.
[4] Chen, Y.P., J.S. Pettis, A. Collins, M.F. Feldaufer. “Prevalence and Transmission of Honeybee Viruses.” Applied and Environmental Microbiology 72.1 (2006): 606-611.
[5] “Cripavirus.” ICTVdB Descriptions <www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.101.0.01.htm>
[6] de Miranda, J.R., M. Drebot, S. Tyler, M. Shen, C.E. Cameron, D.B. Stoltz, S.M. Camazine. “Complete nucleotide sequence of Kashmir bee virus and comparison with acute bee paralysis.” Journal of General Virology 85 (2004): 2263-2270.
[7] “Kashmir bee virus.” NCBI Taxonomy browser <www.ncbi.nlm.nih.gov/Taxonomy/Browser>
[8] Oldroyd, Benjamin P. “Unsolved Mystery: What’s Killing American Honey Bees?” PLoS Biology 5.6 (2007).
[9] Shen, M., L. Cui, N. Ostiguy, D. Cox-Foster. “Intricate transmission routes and interactions between picorna-like viruses (Kashmir bee virus and sacbrood virus) with the honeybee host and the parasitic varroa mite.” Journal of General Virology 86 (2005): 2281-2289.
[10] Slonczewski, J.L., J.W. Foster. “Chapter 6: Virus Structure and Function.” Microbiology: An Evolving Science (2009): 181-217.
Page authored for BIOL 375 Virology, September 2008
