Acidithiobacillus ferrooxidans
Classification
Bacteria; Proteobacteria; Gammaproteobacteria; Acidithiobacillales; Acidithiobacillaceae
Species:
Acidithiobacillus ferrooxidans NCBI: Taxonomy Genome |}
Description and Significance
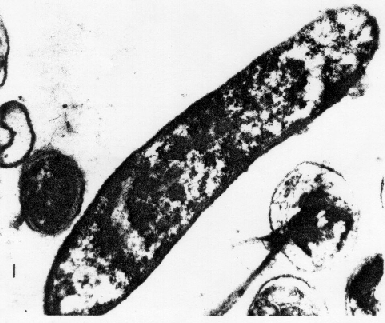
A. ferroxidans is a Gram negative rod shaped bacterium that is commonly found in deep caves or acid mine drainage, such as coal waste (10, 11, 12). These acidophilic bacteria thrive in optimal pH level of 1.5 – 2.5 where they are able to convert insoluble heavy metals to their soluble state. Even low concentrations (ppm) of these metallic ions would be extremely toxic to other bacteria (7, 8). These bacteria have been utilized in industrial bioleaching efforts to extract otherwise unobtainable metals (9).
Genome Structure
Two strains of Acidthiobacillus ferrooxidans have been successfully and completely sequenced. The strains sequenced are ATCC 53993 and ATCC 23270 which consists of genomic sizes of 2.88kb and 2.98kb respectively. Acidithiobacillus ferrooxidans contains a single circular chromosome consisting of around 3,000 genes. The greater majority of species sampled from multiple sites around the world were shown to contain at least one or more plasmids.
Cell Structure and Metabolism
A. ferrooxidans lives in acidic conditions producing a strong reducing environment. The cell is able to maintain homeostasis with a neutral pH of 6.5 within the cytoplasm preventing damage and a reducing environment in periplasm. This allows an increase in redox potential of O2/H2O to 1.12 V at pH 3.2 yielding higher energy when coupled with an electron donor (13, 14). A. ferrooxidans is a chemolithotrophic bacterium which can use many different electron donors to support growth. Because of these multiple pathways, A. ferrooxidans has fairly modest nutritional requirements. In aerobic conditions, electron donors may include ferrous ions or sulfur compounds which are oxidized into ferric iron and sulfuric acid, respectively, yielding high energy (3, 4, 5). However, if oxygen is lacking ferric ions can replace oxygen as the electron acceptor with sulfuric acid donating an electron (6). This pathway yields less energy than aerobic conditions, but energy can still be produced for growth. A. ferrooxidans can fix atmospheric carbon dioxide (CO2) as a carbon source essential for biomass (1). Nitrogen is a common limiting nutrient scarce in the environment; however, A. ferrooxidans can fix atmospheric nitrogen into ammonia (NH3) essential for nucleotides and amino acids (2).
Ecology
....
References
1. Kelly, D. P., and A. P. Harrison. 1989. Genus Thiobacillus Beijerinck, p. 1842-1858. In J. T. Staley, M. P. Bryant, N. Pfennig, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 3. The Williams & Wilkins Co., Baltimore.
2. Mackintosh, M. E. 1978. Nitrogen fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 105:215-218.
3. Pronk, J. T., K. Liem, P. Bos, and J. G. Kuenen. 1991. Energy transduction by anaerobic ferric iron respiration in Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 57:2063-2068.
4. Corbet, C. M., and W. J. Ingledew. 1987. Is Fe3+'2+ cycling an intermediate in sulphur oxidation by Fe2+-grown Thiobacillus ferrooxidans? FEMS Microbiol. Lett. 41:1-6.
5. Sugio, T., K. J. White, E. Shute, D. Choate, and R. C. Blake. 1992. Existence of a hydrogen sulfide:ferric ion oxidoreductase in iron-oxidizing bacteria. Appl. Environ. Microbiol. 58:431-433.
6. Sugio, T., C. Domatsu, 0. Munakata, T. Tano, and K. Imai. 1985.
7. Role of a ferric ion-reducing system in sulfur oxidation of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 49:1401-1406.
8. Rawlings, D. E., I.-M. Pretorius, and D. R. Woods. 1986. Expression of Thiobacillus ferrooxidans plasmid functions and the development of genetic systems for the thiobacilli. Biotechnol. Bioeng. Symp. 16:281-287.
9. Colmer, A. R., and M. E. Hinkle. 1947. The role of microorganisms in acid mine drainage: a preliminary report. Science 106:253–256.
10. Yu Yang, Min-xi Wan, Wu-yang Shi, Hong Peng, Guan-zhou Qiu, Ji-zhong Zhou, Xue-duan Liu. 2007. Bacterial diversity and community structure in acid mine drainage from Dabaoshan Mine, China. AQUATIC MICROBIAL ECOLOGY Vol. 47: 141-151
11. Brierley, C. L. 1982. Microbiological mining. Sci. Am. 247(2):42-51.
12. Merson, J. 1992. Mining with microbes. New Sci. 133:17-19.
13. Sato, A., Y. Fukumori, T. Yano, M. Kai, and T. Yamanaka. 1989. Thiobacillus ferrooxidans cytochrome c-552: purification and some of its molecular features. Biochim. Biophys. Acta 976:129-134.
14. Cox, J. C., and M. D. Brand. 1984. Iron oxidation and energy conservation in the chemoautotroph Thiobacillus ferrooxidans, p.31-46. In W. R. Strohl and 0. H. Touvinen (ed.), Microbial chemoautotrophy. Ohio State University Press, Columbus, Ohio.
15. Jorge Valdés, Inti Pedroso, Raquel Quatrini, Robert J Dodson, Herve Tettelin, Robert Blake, Jonathan A Eisen, and David S Holmes. 2008. Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications, BMC Genomics 9:597
